Operators represent the highest source of contamination inside the cleanroom, both for dry particles and microbe-carrying particles (MCPs); several studies (such as Akers, J. et al., 2004; Ramstorp M, 2000 or Whyte and Hejab, 2007) have demonstrated this.
Today, it is neither technically nor economically feasible to automate the processes to making sterile products so that human operators are no longer needed inside cleanrooms, therefore it is necessary to assess the contamination risks and reduce them to a strict minimum by implementing the required measures.
The draft revision of GMP Annex 1 has defined special requirements to minimise risks of microbiological, particulate and pyrogen contamination during the manufacturing of sterile products. Risk assessments should be carried out to detect, evaluate and eliminate, or reduce as much as possible, any contamination risk.
It makes more sense to eliminate or reduce the risk that the operators represent at the source rather than relying on air filtration or cleaning procedures. Consequently, it is appropriate to define and apply garment selection criteria to manage the risks stemming from both the garment and the operator.
The contamination risks coming from operators wearing cleanroom garments can be divided into two categories:
- The human factor: even when operators are not moving and respecting high standards of personal hygiene, they are shedding every minute hundreds of thousands of non-viable and microbe- carrying particles. Hence, the GMP requirements that no skin may be exposed in the cleanroom manufacturing of sterile products.
The cleanroom garment system plays a crucial role in ensuring that this human contamination is as low as possible. Therefore, it is important to understand and assess the particle retention properties of the garments during the validation process.
- The garment factor: the fabric, manufacturing and washing processes, as well as the handling and the packaging of the cleanroom garment, can introduce contamination into the cleanroom. It is therefore critical to understand and assess the entire value chain of garment manufacturing and handling as well as their performance over time.
Research has shown that every cm2 of our skin hosts millions of microorganisms and that the human body is constantly shedding skin flakes having a size between 0.5μm and 10μm; the cleanroom garments should retain as many of these particles as possible.
Several studies (such as Moschner C. 2011. Ljungqvist B. & Reinmüller B. 2006) have shown that the type of garments worn inside the cleanroom has a direct impact on the contamination risk. Whereas some garments systems have good particle retention properties, others do not. The fabric making the garment, as well as the garment design, (i.e. type of seams, sewing thread, zipper, facial closure) and the manufacturing quality, all have an impact on the filtration efficiency of the garments.
Particle filtration efficiency
To assess the fabric used for making cleanroom garments, it is essential to evaluate the filtration efficiency of the materials used. The particle filtration efficiency (PFE) as per EN 14683, ASTM F2100 and F2299, ISO 11155-1 and EN 143, evaluates the non-viable particle retention (filtration efficiency) of filter media and other filtration devices, at sub-micron levels. The higher the filtration efficiency, the better the barrier to dry particulates and the better the protection of the process.
Data on the particle filtration efficiency of the fabrics used for making cleanroom garments should be part of the risk assessment
The ASTM F2299 works with monodispersed polystyrene latex spheres with a particle size of 0.1 μm that are nebulised, dried and passed through the test material. The particles that passed through the test material are counted using a laser particle counter. This test is mostly used for masks. The ISO 11155-1 uses potassium chloride (KCl) with a range of particle size of 0.3-0.4 μm, 0.4-0.5 μm, 0.5-0.6 μm, 0.6-1.0 μm, 1.0- 2.0 μm, 2.0-3.0 μm, and 3.0-5.0 μm. They are all used with a fixed face velocity. The EN 143 works with sodium chloride (NaCl) with a particle size of 0.3 μm with a fixed flow rate.
All of these tests provide data on the particle filtration efficiency of the fabrics used for making cleanroom garments and should be part of the risk assessment.
The PFE cannot measure viable particles, so it is important to assess the bacterial filtration efficiency (BFE) as per EN 14683, ASTM F2100 and F2101, because it allows the measuring of the percentage of bacteria going through the fabrics.
The BFE test is performed to determine the filtration efficiency by comparing the upstream bacterial control counts to downstream test material counts. A suspension of Staphylococcus aureus is aerosolised using a nebuliser and delivered to the test material at a constant flow rate. The challenge delivery colony forming units (CFUs) is maintained with a mean particle size at 3.0 μm ± 0.3 μm. These bacteria are part of the normal flora of the human body; frequently found in the nose, respiratory tract and on the skin.
The higher the percentage of BFE, the better the filtration efficiency of the fabric. For example, a garment demonstrating a BFE of 90% will provide a better barrier to bacteria and will protect the process from human contamination better than a garment showing a BFE of 50%. This makes the BFE an important element in the risk assessment of the cleanroom garment performance in reducing the risk of human cleanroom contamination.
Fabric and design
Our studies have shown that in both BFE and PFE tests (even for new fabrics) there are significant differences between the various materials used for making cleanroom garments (see Chart 1).
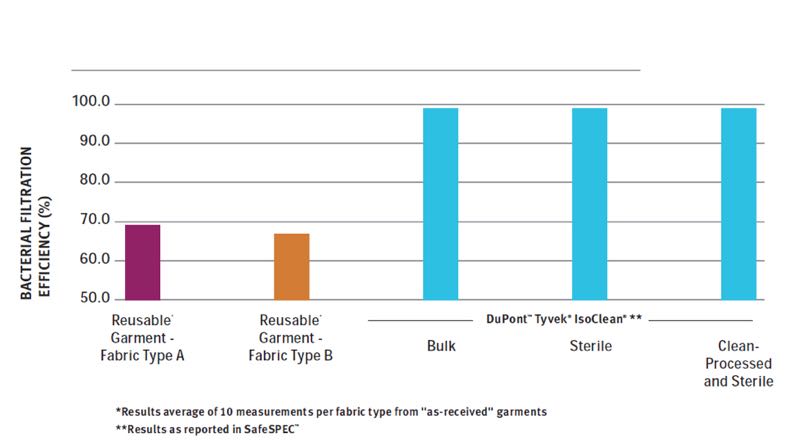
Chart 1. Average Bacterial Filtration Efficiency (%). Higher numbers indicate better filtration efficiency
There seems to be a direct correlation between average fabric pore size (typically between 1 μm for single-use fabrics and 5-6 μm for uncoated polyester fabrics) and both filtration tests. The smaller the pore size, the higher the filtration efficiency seems to be. Both PFE and BFE are measured using swatch samples of fabric only.
However, the material is not the only element influencing the particle retention properties of the garments. The seams, sewing thread, garment design and manufacturing quality play an equally important role. The body box test method (as per IEST-RP-CC003.3) can help in assessing the filtration efficiency of the complete garment system.
The body box test method has been accepted by cleanroom users to qualify the relative cleanliness of a garment system. By testing the particle shed and particle containment of the entire garment system, the body box provides data about overall performance.
The body box test method (as per IEST-RP-CC003.3) can help in assessing the filtration efficiency of the complete garment system
During the body box test, a fully gowned subject is placed in a HEPA-filtered air box and performs a series of activities during a 10-minute test period. These include a double-arm reach activity for 5 minutes, march and slap chest activity for 5 minutes, and doing five deep knee bends in one minute.
Activities are separated by one-minute intervals of standing still. The air is sampled and sent to a particle counter. The data is reported as the average number of particles of sizes 0.3 μm, 0.5 μm and larger per minute during the 10-minute test period. Even though repeated studies have shown that the operator wearing the garments is also influencing the particle contamination, it is possible through repeated series of tests to evaluate the overall particle retention performance of different garment systems (Moschner C., 2011).
As it is necessary to assess the contamination risk each time a cleanroom garment is worn inside the cleanroom, it is recommended to assess the particle retention properties of garments during their entire lifetime.
The studies from Ljungqvist B. and Reinmüller B. (2003, 2004 & 2006), from Whyte, W. and Hejab, M. (2007), and the 2017 study from DuPont (see Chart 2) all show that the filtration efficiencies of reusable cleanroom garments deteriorate over time due to wear-and-tear, as well as washing and sterilisation. All of these studies have shown increased cleanroom contamination after 25 wash and sterilisation cycles: 0.5 μm particles increased by 675%, 5 μm particles increased by 777% and cfu/second by 128% (Ljungqvist B. and Reinmüller B. 2004).
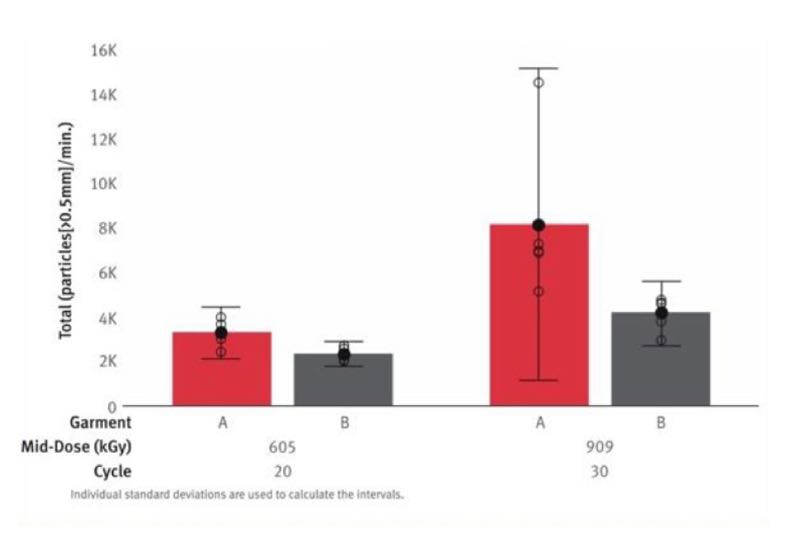
Chart 2. Body Box valuation: sum of shedding for all activities
These performances not only determine how many times a cleanroom garment can be worn, but they also determine the number of the operators that can work inside the cleanroom, based on the air filtration capacity of the HEPA filters used (Ljungqvist B. and Reinmüller B. 2004).
Special care should also be given to the repair procedures of the reusable cleanroom garments; repairs can negatively impact the particle retention performances.
For the risk assessment, it is important to assess the filtration capacity of garments being worn each time operators work inside the cleanroom. As it is impossible to guarantee that all the reusable cleanroom garments have the same filtration properties, it is recommended to take into account the worst-case performances for the risk assessment in order to avoid limit excursions.
Fabric selection
Garments can lead to cleanroom contamination: the fabric can shed particles, the sewing thread may be linting, elastics may shed particles when they are stretched, the garment may not be sterile, etc. All these elements need to be taken into consideration for the risk assessment. The most frequent test method used for evaluating the cleanliness of cleanroom garments is the Helmke drum (IEST-RP-CC003.4.). Garments to be tested are placed into a rotating drum and tumbled to release particulate matter from the garment. An automatic particle counter is used to sample the air within the drum to determine the average particle count during the 10-minute test period.
Results are reported by category. A category I, which is the best possible result according to this test method, requires less than 1,200 particles of >0.5 μm/minute and less than 2,000 particles of >0.3 μm/min.
The cleanliness of garments must be assessed for their entire lifetime. Several studies, such as the 2017 study from DuPont, have shown that the wear-and-tear, as well as the washing and sterilisation, increase the particle shedding of cleanroom garments. It is important to take this factor into consideration for the risk assessment (see Chart 3).
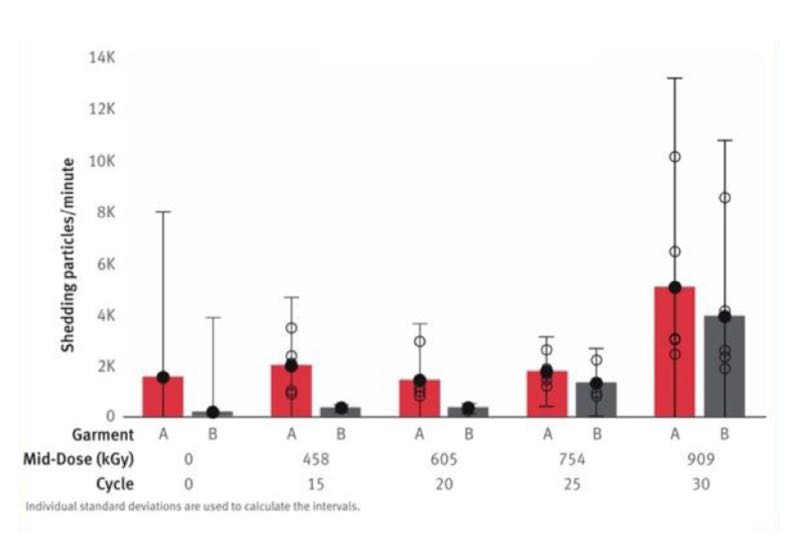
Chart 3. Helmke Drum particle shedding of swatches (≥ 0.5 µm)
Sterility assurance
Another important aspect for sterile manufacturing is to guarantee the sterility of each cleanroom garment used during production. It is therefore recommended that the selected garment manufacturer and/or cleanroom laundry has a validated and documented sterilisation process according to ANSI/AAMI/ISO 11137-1 and that they can ensure a sterility assurance level (SAL) 10-6.
A simple irradiation or autoclaving process or a SAL 10-4 may not be enough to guarantee the sterility of the garments and thus lead to limit excursions.

When the cleanroom operation presents a chemical/biological risk to workers, it is recommended to evaluate protective performance of cleanroom garments as well
Also, an irradiated or sterilised garment is not necessarily a clean garment. The garment manufacturers and/or laundries should also measure the cleanliness of their garments using Helmke drum or body box tests.
One element that is often neglected in the validation process is the packaging the garments come in. It is strongly recommended to make sure that the packaging used is cleanroom validated because it can introduce contamination into the clean environment when the bags are opened.
Holistic approach
To reduce the contamination risk due to the garment, it is recommended to evaluate, assess, validate and audit the entire value chain of cleanroom garments: the fabrics used, the garment conversion workshops, the cleanroom laundry, the aseptic folding and the packaging. The garment maker and/or laundry should supply all requested documentation and guarantee the traceability of their garments through the entire value chain.
During multi-year contracts, it is recommended to assess these elements not only for the initial garment introduction but also for the repairs done and the replacement garments supplied during the full duration of the contract. All the elements listed above must meet the validated specifications during the entire duration of the contract.
It is of the utmost importance to evaluate the benefits and to understand the limitations of different cleanroom garment systems available on the market so as to specify the right solution for the different types of cleanroom applications, whether non-hazardous or hazardous. Companies, such as DuPont, can provide tools and help for these types of risk assessments.
This article is featured in the November 2018 issue of Cleanroom Technology.





