Current containment technologies provide environmental control when completing different operations requiring product or compound protection. But the increasingly potent active ingredients and pharmaceutical compounds developed today require additional protection for the operators and environment. Occupational Exposure Levels (OELs) set by scientific committees and institutes are increasingly strenuous and containment performance can go as low as 10ng/m3, 8 hour Time Weighted Average (TWA).
Containment technologies such as isolator and glovebox fulfill the above requirement at any scale. ISPE defines containment technology as a ‘leak tight enclosure designed to protect operators from hazardous/potent processes or protect processes from people or detrimental external environments or both’ (see Figure 1).
The key priorities here are operator safety and drug quality. When developing new drugs, the risks at early stages are not always fully understood. An ultra-conservative philosophy has to be applied until there is sufficient established data for the product during the process development stage. A common method to establish containment performance is to use a placebo such as micronised lactose with a d50 at approximately 20µg, which accredited laboratories can detect at 2ng.
ISPE guidelines are a useful directive to follow when measuring exposure levels – The ISPE Good Practice Guide: Assessing the Particulate Containment Performance of Pharmaceutical Equipment, for example, provides a scientific risk-based approach. It takes into account background prior to testing and potential weak points around the equipment in addition to passing traffic. It also allows comparable performance.
When validating, tests should be performed at the lower level. A recommended method is to simulate three operations over a one-hour period and utilise this result in a TWA consideration.
In accordance with the British and European standard BS EN 689: 1996 and the British (BOHS) and Dutch (NVvA) occupational hygiene societies’ Sampling Strategy Guidance document, Testing Compliance with Occupational Exposure Limits, statistical analyses of raw occupational hygiene data should be carried out. This represents typically an additional safety factor aiming to control the personal exposures to ≤ 25% of the original required OEL.
How to achieve high containment?
Extreme containment is achieved by considering the following key elements:
Quality of main fabrication and welding. High containment isolators must be built in accordance with ISO 9001:2008 and cGMP quality standards. Internal metallic surface finishing is essential and must be less than 0.4µm Ra to ensure optimum cleanability and avoid product cross-contamination between batches. It is also mandatory in this regard to ensure that all welds are ground smooth, crevice free and polished to match surrounding material. All product contact materials are then to be tested during Factory Acceptance Test (FAT) as well as dye-pen test on welds to ensure tight seal of the enclosure.
Product transfer. All the interconnecting systems have to be integrated by determining the powder handling method through either airlocks cells, rapid transfer ports (RTPs) or bag-out ports. When dispensing or charging large quantities of products, drums can be directly docked into the containment isolator. With increasing potency, drum docking methods have considerably evolved into fully contained drum chambers with automated hoisting system to dock the drum chamber onto the isolator wall, tightly sealed thanks to a pneumatic gasket to ensure that no airborne particles can leak into the room (see Figure 2).
Product extraction is also a key consideration for increased safety with the use of split butterfly valves to off-load powder directly into a container or bottle, or a process vessel. Removable cells are also used to carry the potent product safely from one process to the other. For cytotoxics, transporting product in slurry form is preferred to avoid any explosion or cross contamination. These slurry vessels are filled using an isolator and then dock to the reactor to off-load the slurry safely into a vessel (see Figure 3).

Figure 3: SlurryBox from PSL for cytotoxic slurry charging into vessel
Extraction and air filtration. High containment isolators operate under negative pressure to ensure maximum operator and environment safety. This means that in case of containment breaching, the outside air will be pulled inside the isolator thus avoiding product exposure for the operator. The negative pressure is typically in the region of –100 Pa and is electronically controlled. The isolator can be nitrogen purged for higher explosion risk safety when using solvents.
There is no air exchanged with the surrounding environment. HEPA filters are used to circulate the air within the isolator, with a ‘push-push’ safe remote change procedure allowing the operators to stay protected even when changing the filter through the glovebox.
A pre-filtration mesh can also be fitted in front of the extraction HEPA filter assembly to increase containment level. An oxygen meter sensor can be located on the extract line to monitor the oxygen level within the isolator and report the data to the PLC.
Cleaning methods. Cleaning regimes form an integral part of reducing the risk of cross contamination. The use of wipes to pre-clean is preferred, as it provides a concentrated solid waste rather than high volumes of liquid waste, which are more costly to treat. Clean-in-place (CIP) is then completed following the wiping technique. Product contact surfaces have to be carefully assessed to ensure optimal cleaning operations.
Process equipment integration. To achieve higher containment and reduce the risk of exposure and cross-contamination, entire process lines are now integrated fully into isolators and gloveboxes. Having the equipment fully contained and not simply the transfer methods (charging and off-loading) allows better control of the environment, all the equipment assembly can be cleaned safely while the overall footprint and capital investment for a new production line can be greatly reduced.
This trend is seen more often with small-scale production such as clinical trial manufacturing as the process systems are compact and easy to integrate. Larger scale production lines are, however, more complex to integrate and require high levels of expertise and collaboration between the OEMs and the containment provider.
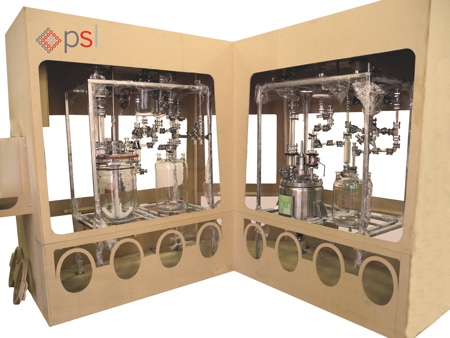
Figure 4: Full scale mock-up for ergonomics review
To ensure a smooth and efficient integration of several pieces of equipment within an isolator, a full scale mock-up should be built. A front-end design study using a full scale model of an isolator is useful to ensure that every connection and product transfer method between all of the integrated equipment has been planned. But more importantly, a mock up survey can allow for verifying and refining the ergonomics (see Figure 4).
When operating several pieces of process equipment inside a glovebox, many parameters have to be considered to ensure that the operator can safely and easily operate the systems. Because of the solid barrier, usually an acrylic window protecting the operator, there is a constraint with the ergonomics. Some operations can also be difficult to perform using gloves. Integrated process equipment may have to be redesigned for better manoeuvrability and the location of the glove ports may change. During the front end design review, cleaning is also a key factor to consider with regard to the ergonomics of the isolator and integrated process equipment.
Case study
The following case study illustrates an example of a high containment facility designed to safely control cytotoxic exposures during the production of antibody drug conjugates (ADCs).The production line comprised the process steps of reaction, filtration and drying and each step required the use of isolation technology to protect the product, the environment and the operators from the potent ADCs (see Figure 5).

Figure 5: Installed clinical trial isolator for high potency ADCs manufacturing
A full high containment suite was required to integrate efficiently and safely the synthesis process equipment used in the production, such as dissolution vessels, reactors and filter vessels. The isolator suite also contained tray dryers for vacuum drying as a final step. The facility had to guarantee an OEL of less than 20ng/m3 on 8h TWA.
After installation, OEL tests were performed on site by a third party industrial hygienist and results exceeded the levels requested, with a 15ng/m3 8h TWA exposure in the finishing drying chamber and less than 2.7ng/m3 8h TWA to non-detectable exposure in the crude drying chamber.
Figure 1: Reactor Charging Isolator for nano-containment of less than 10ng/m3 8h TWA Figure 2: Contained drum chamber with automated hoist system for highly contained reactor charging Figure 3: SlurryBox from PSL for cytotoxic slurry charging into vessel Figure 4: Full scale mock-up for ergonomics review Figure 5: Installed clinical trial isolator for high potency ADCs manufacturing




