The packaging and dispensing of liquid ophthalmic preparations has traditionally presented a number of challenges with regard to preserving the sterility of the medication and preventing contamination while still allowing the patient to open the pack and administer the product accurately and at the correct dose.
According to G Dinakaran, Chairman and Managing Director of Doctor Pack India, based in Bangalore, there are three main options widely used today. The first is a container/closure system consisting of three components: a squeezable plastics bottle; an open nozzle and a cap. The second is comprised of two components: a squeezable plastics bottle with a sealed nozzle manufactured using blow/fill/seal (BFS) technology; and a closure that incorporates a method of piercing the nozzle.
The final option is another three-part container/closure solution consisting of a squeezable plastics bottle, a closed nozzle and a piercing cap. The second and third options are deemed preferable, especially in developing countries, as the product is hermetically sealed within the container until the diaphragm is pierced for dispensing. The closed container system eliminates the risk of sterility failure or leakage due to improper capping in the assembly machine; in the first option, which has an open nozzle, the seal integrity depends on the capping torque and the final capping seating position above the nozzle hole. Even a slight cross capping can cause the leakage as well as loss of sterility.
Unsuitable tools
But once the product is in the hands of the end user, the sealed nozzle options present new challenges because to use the medication the patient must pierce the diaphragm. After the medication is filled and sealed on the BFS bottle, or filled and sealed with the closed nozzle, a conventional piercing closure is half capped onto the bottle thread. The patient must first tighten the cap fully down to pierce the diaphragm to enable the medication to be dispensed.
The patient is likely to resort to any available tool such as a non-sterile pin or needle or scissors to pierce the diaphragm
The conventional piercing cap has several drawbacks. The first is that the end user is confused by the need to tighten the cap down before unscrewing it as this is contrary to standard practice. Finding that the bottle is still sealed after the cap has been removed in the conventional way, the patient is likely to resort to any available tool such as a non-sterile pin or needle or scissors to pierce the diaphragm, thereby contaminating the dispensing tip as well the content and posing a serious threat to the eye.
The second disadvantage is that the drops that are dispensed by such packs will have variable microlitre dose delivery depending on the squeeze force applied by the patient. This will lead to over- or under-dose delivery. Not only can the overdosing of eye medication such as steroids and antibiotics have serious side-effects and consequences, it also results in wastage and increases the cost of the treatment.
The third drawback, during filling and assembly of the piercing cap onto the bottle, accidental pre-piercing of the nozzle can lead to leakage in the market as well as rendering the content non sterile.
With a view to overcoming these hazards, Doctor Pack, a leading packaging and medical device manufacturer based in India, has developed Magic Cap – an innovative alternative to the non user-friendly conventional piercing cap used in the packaging of eye, ear, nasal and oral drops. It has an automatic mechanism to pierce the sealed diaphragm of the bottle or the nozzle, just by opening the cap for dispensing. Furthermore, it delivers controlled microlitre drops.
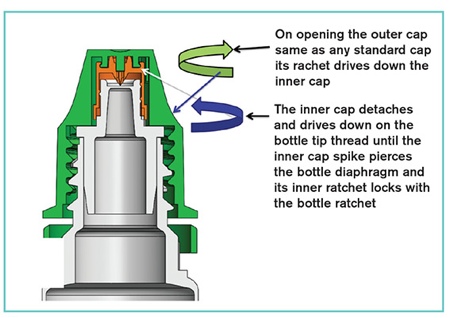
Manufactured in a validated cleanroom, the Magic Cap consists of an outer and inner cap that are preassembled and supplied as a single component. Once the bottle has been filled and sealed, the closure is fully capped onto the bottle. It is suitable for use on standard capping machines and there is no chance of pre-piercing of the diaphragm during high-speed assembly; it can also be supplied as a gamma sterilised cap.
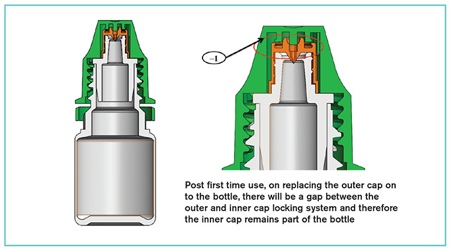
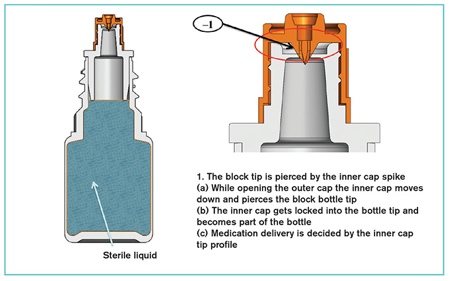
The inner cap has a reverse inner thread, an inner ratchet to lock onto the bottle ratchet while in pierced condition, a piercing spike, and an outer ratchet that is driven by the outer cap's inner ratchet on opening of the outer cap during first use. When the patient removes the closure by unscrewing the outer cap, the reverse thread pushes the inner cap downwards to enable the spike to pierce the diaphragm and the medication to be dispensed.
The design profile of the inner cap delivers a consistent drop count and minimises under- and over-dose variations. This in turn reduces wastage and makes the product more economical.
The closure is simple and easy to operate in the same way as a standard screw cap, and requires no special instructions to be given to the patient. The pilot tool Magic Cap samples were tested on 200 bottles by three different groups of people in different age groups. All of them were able to open the bottle and dispense the medication without any secondary operation to pierce the nozzle diaphragm.
After the successful product design validation and pilot tool development, Magic Cap samples were injection-moulded in one of Doctor Pack’s cleanrooms. A number of pharma companies are currently carrying out comprehensive performance studies using the Magic Cap and feedback has been very encouraging, said Dinakaran.
The company is in the final process of patent submission and expects the product to be commercially available on the international market from April 2014.
Doctor Pack India specialises in the design, development and manufacture of primary packaging and drug delivery devices. It has the following approvals: ISO 15378 , ISO 13485 and ISO 14001 , Type III DMF and CE Marking. Cleanroom manufacturing is at the heart of the company. Its state-of-art facility is equipped with advanced machines, including blow-moulding, injection blow-moulding, micro-moulding, automated assembly lines and printing machines, in an ISO 14644-1 to 5, cGMP compliant, validated cleanroom.
Products are sterilised at the company’s service provider, having Type V DMF and ISO 13485 Certified gamma sterilisation and ETO sterilisation plants. All these facilities are validated with the actual products in accordance with ISO 11137 and ISO 11135.
Magic Cap was a runner-up in the CPhI Pharma Awards in October 2013 in the Packaging Innovation category. ‘We strongly believe the Magic Cap will bring a lot of benefits to the end user, such as safety, ease of use and fewer side-effects,’ Dinakaran said. ‘Doctor Pack is more than just a packaging company. We provide comprehensive support to a product’s lifecycle from innovation through commercialisation via patient-centred delivery solutions.’




