Years of research into polio and the production of a vaccine have led to an almost worldwide extinction of the poliovirus. However, the now used and stored samples of the virus in research facilities form a potential risk for the reintroduction of polio.
The World Health Organization (WHO) has set up a plan to reduce the total number of research facilities that use the poliovirus to a limited number of certified research facilities. These “essential locations” must comply with specific measures to reduce the risk of polio reintroduction to a minimum.
These research facilities can only be certified when all the risk mitigation measures are implemented correctly. This article elaborates on these measures and indicates how they have to be implemented.
Polio is well known as an infectious disease against which, in large areas of the world, every citizen is vaccinated against. The virus spreads through the faeces of infected people.
This happens under circumstances relating to poor hygiene. Of the three existing polio types, type 2 is the most infectious.
Current situation
Polio is extinct in the Western world. Afghanistan, Pakistan, India and Nigeria are the last countries where polio has recently been endemic. India was declared polio free in 2012. At the moment, polio is still active in Pakistan, Afghanistan and Nigeria (where it reappeared in 2016).
There is a theoretical risk of the reintroduction of polio worldwide for the following reasons:
- Polio is still active in the countries mentioned previously
- The poliovirus is used for research purposes at different worldwide locations
- Vaccines against polio are produced at different worldwide locations
IPV and OPV vaccine
Two types of polio vaccines are available today; the Inactivated Polio Vaccine (IPV) and the Oral Polio Vaccine (OPV), named after the required manner of administration. The IPV (also known as the Salk vaccine) contains an inactivated (dead) virus that is administered via injection. The OPV (also known as Sabin vaccine) contains an attenuated (reduced in virulence) but still alive virus. This virus can multiply inside the human body but will not cause a disease.
The OPV has some downsides: people with an impaired immune system may get a severe infection and the attenuated vaccine virus can then evolve back to the wild-type virus and actually cause paralysis. Until recently, most countries worldwide used the less expensive oral vaccine. Since polio is becoming rare, the downsides of the oral vaccine are starting to outweigh the benefits and the richer countries, in particular, are switching to the Salk vaccine.
The goal of GAP III
Despite the fact that polio no longer occurs in large areas of the world, there remains a risk of reintroduction caused by improper storage, misuse or incorrect action. To reduce this risk, a Global Action Plan (GAP III) has been set up by the World Health Organization (WHO). The first version of GAP III was released in 2009. Currently, the third edition is available which includes the latest insights, designed to provide a proper balance between the population risks and the activities needed to produce the vaccine.
The reduction in the total number of locations where the polio type 2 virus is used is seen as the first priority of the GAP III. The starting point is to destroy as much of the type 2 material as possible and to only leave enough material available to produce vaccines.
In the GAP III, a plan was drawn out to limit the number of locations where the poliovirus is used/stored to a small number of specifically dedicated locations. By restricting the number of locations and, through better furnishing and securing of the dedicated locations, the risk of an outbreak originating from a research facility can be greatly reduced.
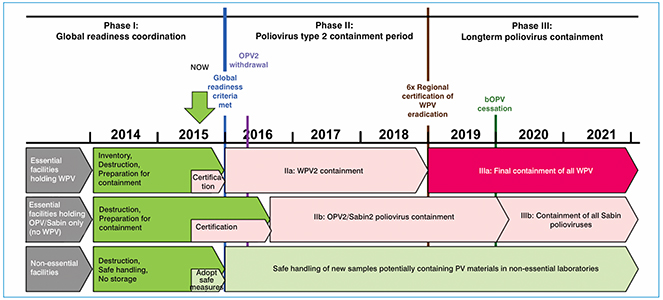
Fig. 1. Timeline of the implementation of GAP III
GAP III implementation timeline
The GAP III action plan consists of the following steps:
- Identifying the current locations of polio vaccines
- Reducing this number of locations
- Selecting the locations where the polio type 2 virus is allowed by a national authority (Ministry of Health)
- Destruction or isolation of the virus at locations where the virus will not be used anymore
- Authorised locations will have to meet stringent requirements as stated in the GAP III
- Certification of the selected locations on the requirements stated in the GAP III
- A Risk Assesment is part of the certification (Annex 2 and 3)
- National authorities will need to check compliance with these requirements
The GAP III offers a set-up of a Bio Risk Management System which can be used as framework for the facilities that are selected as “essential”. It explains how to deal with the poliovirus and its storage. In the Bio Risk Management System, for 16 elements the requirements are defined for:
- The design
- The performing of operations
- The responsibilities of the management
In the Bio Risk Management System risks and measures should be defined for:
- The available materials
- The risks for the operators
- Requirements for education and training of operators
- Working procedures
- Workwear
- Awareness of the operator
- Risks of injury
- Emergency situations
- Registrations of incidents
- Changes in the previously assessed set-up of the lab
- Measures for regular maintenance
- Procedures for disinfection and waste processing
- Internal and external transport
- Security and access control
A key aspect of the Bio Risk Management System is the idea that all systems must be validated. In practice, this means that existing files need to be checked and changed. In some cases, the existing systems need to be replaced by systems for which validation is possible.
What are the consequences of GAP III on the fit-out of the laboratory?
In essence, the requirements for a research facility where the poliovirus is used, are similar to the requirements for a BSL 3 laboratory according to the WHO Laboratory Biosafety Manual, third edition 2004. In addition to the requirements for the primary and secondary measures, additional measures are listed in GAP III.
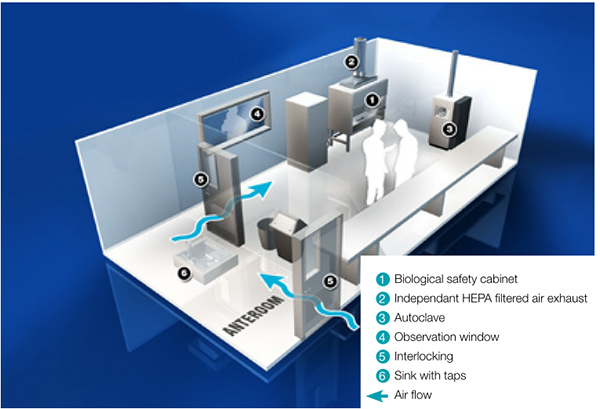
Fig. 2. BSL3 Laboratory – sealed for gas decontamination
What is meant by primary measures?
The primary risk of human beings and their surroundings coming into contact with the virus are reduced by using a class 2 or 3 biosafety cabinets. Validation of the proper functioning of the cabinet is an absolute requirement. The principle of a class 2 biosafety cabinet is illustrated in figure 3. In such a cabinet, a sterile working environment is created by using a downflow of air through a High Efficiency Particulate Air (HEPA) filter. The operator and the surroundings are protected by an inflow of air through the frontal opening. This system has to comply with the EN 12469 standard.
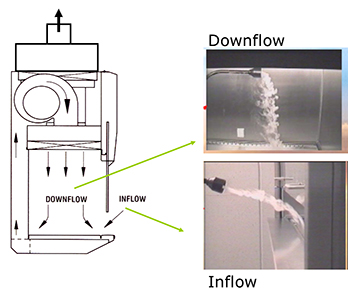
Fig. 3. Class 2 biosafety cabinet
Secondary measures
There are also a number of secondary measures that apply to a standard BSL 3 laboratory, including disinfection.
To ensure a validated disinfection it is a required that the room can be completely sealed. Special attention should be given to the complete sealing of the wall sockets and of areas where industrial materials may penetrate the walls. A negative pressure in the room during disinfection is preferred.
All incoming and outgoing materials go through an autoclave. A gas sluice with a validated disinfection system can be used for materials that cannot be autoclaved.
Materials that are transported from the biosafety cabinets to other locations in the laboratory have to be immersed in a disinfection liquid in a so-called dunk tank. Below are some examples.

Fig. 4. Example of an in- and out-airlock with disinfection shower
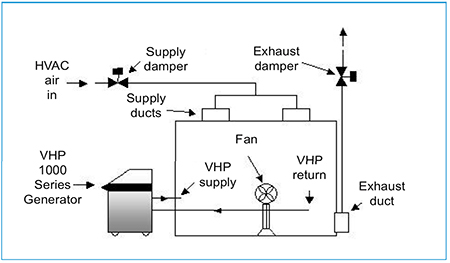
Fig. 5. Room decontamination
Additional measures
The following measures will be implemented in phases according to the schedule shown in Table 1:
- Supply ventilation system with backflow security
- Exhaust ventilation with HEPA filter
- Gas-tight channel system with regards to the possibility of gas disinfection
- Directional airstream
- Monitoring/warning of the directional airstream
- Complete sewage system, including outgoing shower, eyewash and non-sterilised condensation water of the autoclave, must be connected to a validated kill tank system
- All industrial material, such as gas, water and drains should be provided with backflow security
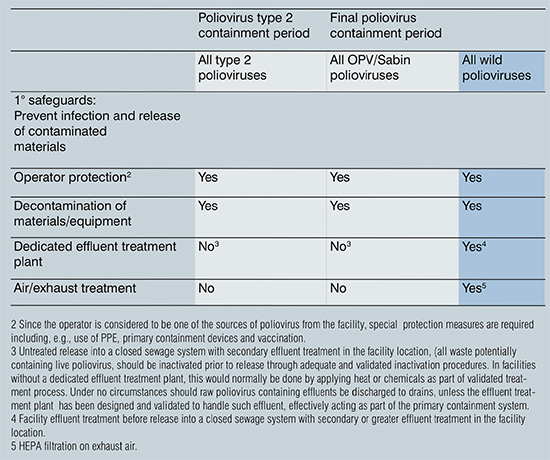
Table 1: Overview of measures according to time schedule
The “essential” facilities are monitored and certified by the national authorities (National Authorities for Containment or NAC). The compliance monitoring for any measures concerning the GAP III are the responsibility of the NAC.
Conclusion
The GAP III gives a description of the phasing and implementation of the measures to reduce the total number of facilities where the polio vaccine is used or stored. For the locations selected as “essential”, it is necessary to start early with a inventory of the measures that are needed in order to meet the GAP III. The annexes included in the GAP III can support this. The conditions for the facilities that desire certification are stated in annex 2 and 3. Verification methods for the certification of essential facilities are presented in annex 4.
When designing or modifying an “essential” facility, it is wise to take account of the preparation and/or implementation of the additional measures beforehand (see time schedule). Facility-specific measures may be required, these depend on the results of the Bio Risk Management Tool.
Information sources
- WHO - GAP III - 2014 www.who.int/immunization/sage/meetings/2014/october3_GAP_III_Revision_10Oct14.pdf
- WHO – containment key points January 12, 2016 www.polioeradication.org/wp-content/uploads/2016/07/Containment_keypoints_18Feb2016.pdf




