Laurent Leblanc, products manager for Biopharma Culture Media at bioMérieux, examines the crucial role of Media Fill Tests in assuring the sterility of pharmaceutical processes.
Production of parenteral drugs is considered one of the most complex processes in the pharmaceutical industry. To ensure sterility of the products, manufacturers use an aseptic filling process and must prove that this practice is under control using a Media Fill Test (MFT).
European and US regulatory agencies describe MFT as a standard approach for validating aseptic filling lines. Qualification of a line requires three successful MFTs in a row, in addition to a periodic re-evaluation of lines at least twice a year. Resources dedicated to this operation could be considerable, depending on the number of units to inspect.
However, MFT is indispensible for guaranteeing the manufacturing process will remain under control. Not only does it simulate manufacturing conditions, but it also tests their robustness by evaluating the worst-case production conditions, such as planned interventions of operators or the number of authorised actions in a protected environment.
The essential element of the MFT is the culture media. Trypcase Soy Broth (TSB) is the medium traditionally used for performing simulations of aseptic procedures. It is a rich media that fosters the growth of the main aerobic bacteria as well as yeasts and fungi.
The selection and validation of this culture media is critical for pharma companies. Its performance governs the success of the MFT and then whether or not a manufacturing line will be used. Various notions must be taken into consideration, such as growth promotion of micro-organisms in a stressful environment and the practicability of an application that remains limited by multiple constraints.
The selection and validation of culture media is critical for pharma companies: its performance governs the success of the MFT and then whether or not a manufacturing line will be used
An investigation conducted by the PDA1 found that 62% of pharma companies filled more than 5,000 units for each MFT, and about 25% filled more than 15,000 units. Although these units may vary in terms of size, fill volume and transparency, they must all be inspected individually and manually to detect any contamination, no matter how small. Reading a MFT result is a delicate exercise and can be prone to human error.
The study therefore introduced an innovative solution that reinforces the MFT reading experience. Developed by the r&d laboratories of bioMérieux, the culture media TSB 3P with Vegetable Peptones is dehydrated, cold filterable, double-wrapped and irradiated at a minimum of 25kGy; the formulation uses vegetable peptones, without animal raw material. The media offers a growth-based colour indicator as well as an optimised formulation for the growth of a broad range of environmental micro-organisms studied under aerobic and anaerobic conditions.
The first role of culture media is obviously to foster the growth of micro-organisms. The ingredients used in the formulation are mostly of biological origin, and are therefore particularly sensitive and variable by nature. For example, vegetable origin peptones are inherently subject to climatic variations, daylight, rainfall etc., which can lead to slightly different properties between lots.
>the culture media must be able to closely mimic production at all steps of the manufacturing process
A media intended for aseptic process simulation must be “wide-ranging”, meaning it would allow a minimum of growth for all microflora susceptible to being found in a production environment. In addition, the culture media must be able to closely mimic production at all steps of the manufacturing process: preparation in unheated tanks and cold sterilising filtration are properties generally required to allow optimal use of an MFT. The selection of raw materials for TSB 3P Vegetable Peptones with a colour indicator was motivated by the following criteria: the absence of mycoplasma, low bioburden, good filterability and excellent growth performance of vegetable peptones.
Growth promotion study: The vegetable peptone media TSB 3P with colour indicator is irradiated at between 25 and 40kGy. With the culture media under-going extreme conditions during irradiation, the first question is to determine the impact on the growth promotion of the media regarding micro-organisms. A verification of the growth promotion before and after irradiation was conducted. It was measured based on a scale of turbidity on the strains described in Pharmacopeia but also on a panel of wild strains found in pharma-ceutical cleanrooms. All of the inocula had a concentration of less than 100 CFU per tube. A total of 101 micro-organisms were evaluated: 80 aerobic bacteria, six anaerobic bacteria, six yeasts and nine fungi.
The results shown in Figure 1 clearly prove the absence of visible effect from irradiation on microbial growth but also the excellent growth promotion of the media for the micro-organisms tested; the turbidity observed was vastly higher than that of the non-irradiated TSB standard. The greater the turbidity, the easier it is to detect contamination during reading.
TSB 3P was developed to foster the growth of both aerobic and aerobic organisms. A growth promotion study was therefore conducted on a panel of anaerobic organisms (see Figure 2). Where classical TSB does not allow the growth of certain anaerobic organisms, TSB 3P fostered a growth of 100% of the panel tested, even allowing an early growth well before the 14 days of incubation, at a temperature of 20-25°C. The bacteria Propionibacterium acnes ATCC 6919 was also detected at four days of incubation at 20-25°C. This bacterium is frequently isolated from human skin. In fact, 70% of the potential sources of contamination of aseptic process simulations come from the personnel;1 its early detection is an asset in the application of an MFT.
Color indicator performance study: The TSB 3P culture media presents excellent fertility over a wide variety of micro-organisms. This capacity gives it the properties of wide-ranging media conforming to the expectations of a culture media in MFT, also allowing the growth of aerobic and anaerobic bacteria, yeasts and fungi.
The TSB 3P culture media contains a colour indicator that gives it a fuchsia (pinkish-red) colour once reconstituted. The metabolism of the micro-organisms growing in the broth leads to the reduction of this compound and the disappearance of the colour. The indicator was selected for its capacity to be metabolised by a very large spectrum of micro-organisms and for its property of irreversible discoloration after reduction by microbial growth.
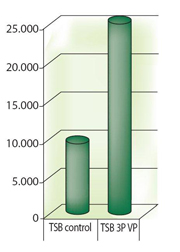
Figure 2: Growth intensity obtained with different anaerobic organisms tested anaerobically after 8 days of incubation at 20-25°C
The colour change was individually assessed for each of the 95 aerobic strains. The Redox nature of the indicator leads to a spontaneous change of colour in an oxygen-free atmosphere (anaerobic). The study was then conducted on a panel of aerobic organisms: out of the 95 strains tested, 92 discoloured the culture media. Only three slow-growth micro-organisms did not discolour the indicator after 14 days of incubation: Methylobacterium meso-philicum 0306751; Corynebcaterium jeikeium 8710127; Kocuria rosea 005008. However, the turbidity remained clearly visible.
The other strains of Corynebacterium and Kocuria tested still metabolised the colour indicator with the irreversible colour change. The vast majority of micro-organisms tested (97%) metabolised the indicator in the incubation conditions of an MFT after 14 days at both 20-25°C and 30-35°C. By being a well-adapted growth indicator in the application of MFTs, the universal Redox characteristics of the media dye make it possible to be reduced by the metabolism of micro-organisms.
Mycoplasma absence: The term mycoplasmas is the commonly used to designate the mollicutes group; which are animal and plant cell parasites. Mycoplasmas are characterised by a lack of cell wall and are physically small – less than 1µm – which gives them the ability to pass through validated filters used to stop bacteria and other yeasts or fungi. Their presence is undesirable in a culture medium coming into contact with a production line.
Validation for the absence of mycoplasma was conducted by measuring the natural burden of contaminants present in the peptones before irradiation but also by validating the reduction of biological indicators by the irradiation cycle (minimum dose of 25kGy). A laboratory specialised in mycoplasma research analysed the different samples of media by using two different techniques: an indirect culture with Vero indicative cells and a genetic amplification detection method (PCR). The analysis showed that the raw material selected had a total absence of mycoplasma. The irradiation cycle at a minimal dose ensured a reduction of more than 8 Log10 mycoplasmas, which ensures the final cleanness of the media. This study presented an innovative solution for improving the precision of MFT reading and also reduces the risk of errors.
In conclusion, MFT are an essential part of the validation of aseptic processes and ongoing periodic evaluation. The adoption of new higher performance processes, makes it possible to manufacture lots containing hundreds of thousands of units, calling into question the relevance of the 15,000 unit limit described in Annex I of Good Manufacturing Practices.2 A more mathematical approach to aseptic process simulation estimates that each MFT would theoretically need to be run on as many as 100,000 to 400,000 units to be able to detect a very low contamination rate.3
With an ever-larger number of units filled, what does the future hold for MFT manual readings? Will this test become a production bottleneck for pharmaceutical companies? It is clear that a growth in the number of units to control will require more time and resources, but the risk of errors will also increase. Innovations will, therefore, be indispensible to make it possible to simplify reading. Certain companies are now seeking solutions to automate reading, for which a growth indicator would be of unquestioned benefit.
References
1. PDA Technical Report No 36, Current Practices in the Validation of Aseptic Processing – 2001. PDA Journal of Pharmaceutical Science and Technology, May-June 2002; supplement TR36, Vol. 56, 3
2. Guidance for Industry – Sterile Drug Products Produced by Aseptic Processing – Pharmaceutical cGMPs, FDA 2004
3. Nigel A. Halls, Practicalities of setting acceptance criteria for media fill trials; PDA Journal of Pharmaceutical Science and Technology, 200; 54, 247-252




