The internationally renowned Erasmus Medical Centre in Rotterdam is using an integral approach to build for its future. The university’s medical centre, which offers academic medical care, medical education and scientific research, is becoming the biggest ‘drive-in’ hospital in the world, with quick and comfortable accessibility. However, it was decided to move the drug manufacturing department in the hospital pharmacy (where a great diversity of drugs is prepared for some very specific patient groups) to a more central site in the country, freeing it from both the technical and space limitations that were present in the existing building.
‘As an academic hospital, we want to maintain our own control of research, education and care for the benefit of specific patient groups,’ says Dr András Vermes, Erasmus MC hospital pharmacist and head of drug manufacturing at Erasmus MC. ‘This also means supplying children, who represent a relatively small patient group, with the requisite medicines they need but which are not commercially available.
‘In the past few years many public pharmacies stopped making their own preparations because alternative, large-scale manufacturers offered competitive prices. We, as hospital pharmacists, fill the gap created in the area of research that is not commercially attractive, but which is necessary for effective customised treatment and therapy.’
Erasmus MC is not only looking to anticipate future pharmacy developments with the construction of the new hospital pharmacy; efficiency is also a key issue. Not every hospital will be permitted or able to build pharmaceutical manufacturing capacity exclusively for itself. It is, after all, funded by public (insurance) money. Moreover, hospital pharmacists must meet the same, ever stricter, industrial standards that commercial research and production laboratories do. Prompted by a vision, unique in Dutch terms, Erasmus MC has located the new production pharmacy more centrally in the country, next to the A15 highway, so that collaboration with and supply to other hospitals will be better facilitated.
EGM architecten was approached to design the new building. The architectural firm is renowned for setting trends in the area of design and construction for healthcare, and has been responsible for the spectacular metamorphosis of various medical university complexes, including the Erasmus Medical Centre in Rotterdam. Royal HaskoningDHV, one of Europe’s leading engineering firms, was called in to design the technical building services. The A15 Pharmacy consists of a production building and a warehouse. The production building includes 1,900m2 cleanrooms, 400m2 laboratories, 800m2 technical rooms and 900m2 offices. The storage and distribution warehouse takes up 300m2.
An important aspect of the design is the abundance of daylight present in the interior, a major factor contributing to the employees’ well-being. Large glass surfaces, wide corridors and long sightlines stimulate communication and interaction among employees.
The building derives its shape and appearance from the site and its function. The narrow construction site and the desired production process have resulted in an elongated building in which the functional structure was designed in such a way that it enables an optimum production process. The external design is analogous to the building’s function – with smooth, flat outer walls as a reference to the sterile process that takes place within. The external walls are clad with compressed fibre cement sheeting made of recycled material, that is 100% recyclable at the end of its lifespan – one of the many sustainable aspects of the building.
Box within a box
Strictly speaking, a cleanroom is nothing more than a ‘protected and protecting’ space – a box in which products can be prepared and tested under controlled conditions. Such rooms must comply with the strictest requirements as far as hygiene, smoothness and flatness of materials and purity of the air are concerned. Anything that does not necessarily have to be placed inside the manufacturing area should preferably be available in a shell around these rooms. This creates a peripheral space containing the ‘technical service facilities’ that provide optimum support to the work process in the cleanrooms, enabling maintenance to be performed without interfering with the primary process.

Large glass surfaces provide abundant daylight
These service facilities have to be as accessible as possible to make it easier and thus cheaper to provide a good maintenance service. In cleanrooms, this is often a difficult task due to construction elements such as the ceiling suspension system, which is in the way of the technical engineers, who prefer an empty space for pipes and ducts.
As a result, when designing the A15 Pharmacy, a peripheral space around the cleanrooms was used and applied as a box-in-box principle. Additional space was created for a services distribution floor, consisting of a walkable cleanroom ceiling spanning the entire cleanroom area, located between the technical equipment floor and the cleanroom floor. This extra space allows for distribution of all technical utilities with easy access to the grids and fittings of the rooms below. All central technical building services are located in rooms directly above this distribution floor.

The intermediate service floor
Such a distribution floor is unique. After all, it is extra space, which costs additional money. This also applies to the wide corridor that surrounds the cleanrooms and thus separates them from the rest of the building and the outside world.
According to Dr Vermes, the services floor and the corridor do cost ‘additional bricks and paint’ that are not directly involved in production operations, but it is a relatively limited investment in relation to the total cost and the advantages are great. The corridor around the rooms provides a view of the production process without the need to enter the department. And thanks to the services distribution floor, all maintenance and repair work on the technical components can be carried out from outside the manufacturing area, thereby safeguarding the hygienic conditions in the cleanrooms. Also, adaptations and modifications can take place during normal working hours rather than (expensive) evening or weekend hours. This guarantees optimum non-disruptive operational management and virtually zero downtime for the production process.
The box-in-box principle also offered opportunities for optimising fire compartmentalisation as well as a different approach to achieving the required flow-pressure cascade. The ventilation system is arranged in a modular way, using main ducts as header and active flow and temperature control per room as modules, all located in the technical room, as is good practice in cleanroom design.
3D integral design
Integral design is a necessity when designing laboratories and cleanrooms. Making a technically and functionally optimum building is a collaborative task for the client and the architect, as well as the technical and construction engineer. This requires people who are bold enough to see beyond the boundaries of their own field, who have a good understanding of the importance of one another’s disciplines and are willing to meet half-way – people who want to search for the best solutions together.
The Building Information Model (BIM) is an indispensable tool for this as it presents all components in the design three-dimensionally and shares them with suppliers, contractors, managers and the client. From the very first design stage the various design disciplines have the opportunity to optimally integrate their ideas and input into the design.
Buildings with a high standard of technical building services can be designed in a completely integrated way by using BIM. This contributes to realising a product that is as efficient as possible from a technical and functional point of view. BIM also offers the opportunity to keep design errors and failure costs during construction to an absolute minimum. Considering the huge investments required for the construction of laboratories and cleanrooms, this is a major advantage.
The Building Information Model
EGM architecten regards the BIM as a crucial means of communication during design, construction and operation of a building. It breaks down the barriers between the various disciplines and stimulates a mutually positive effect on designers, engineers, builders and the client, who will see faster results. During the design process, the model can make calculations of, for instance, construction and other costs, construction time, energy consumption, including the effect as a result of design changes and variants. Since all designers and engineers work together in one model, design errors can be detected at an early stage, and the schedule and budget can be monitored properly. The 3D model can also generate all necessary 2D drawings.
EGM architecten has developed a protocol for working with BIM in which agreements with technical engineers and designers are set down in advance. Clients and users can also gain easy access to the model through a viewer and can thus be actively involved in the design process. Among other things, they can record observations and communicate them to the architect.
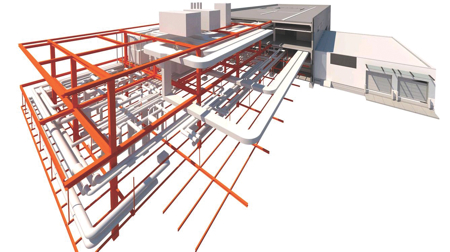
Working with BIM is still in a relatively early stage, even though EGM architecten and Royal HaskoningDHV have many years of experience with it. If all changes are updated correctly every time, a digital building model is created that corresponds exactly with the actual building. This may benefit a client in the management phase. BIM has already proven its great added value in the design phase of the A15 Pharmacy.
EGM architecten pays a great deal of attention to integrating the building structure and technical services in the design phase. This co-ordination is even more crucial when designing a building with such technical facilities as required by the A15 Pharmacy.
‘As an architect, you naturally want to end up with a beautiful building, but it is also my job to create an environment where people can do their work pleasantly and safely, and that contributes to their well-being. From this vision, I strive for that beautiful building. But that is possible only if the building is completely in order on a functional level,’ says Gerard de Jong, architect at EGM.
The insight provided by BIM during the design process can also relieve the natural tension between the work of the technical engineer and the architect. ‘We demand a great deal of space for technical equipment and infrastructure located in the most efficient place. An architect particularly wants the design to turn out to be a beautiful building. This contrast often causes clashes, but that was never the case in the design process of the A15 Pharmacy,’ says Henk Kunst, engineering consultant/project manager at Royal HaskoningDHV.
The digital BIM provides immediate insight into the consequences of choices – for instance, in finding the most suitable location for the cooling units. The roof is often an inevitable solution for the technical engineer, but for the architect this is a component that detracts from his design. The two parties positioned the cooling units together in the BIM model, and decided on the sightlines so they could give the unit an optimum, fairly inconspicuous place on the roof.
‘There is no point in blocking things that later turn out to be inevitable,’ de Jong adds. ‘The faster you search for an alternative solution, the more flexible you keep the process.’
The attitude of obtaining an optimum result, together, spread from the design team to the construction team and the building contractors also looked to create solutions together, rather than having the often defensive attitude of contract management.
Because the architect and engineers co-operated intensively in the BIM model, they managed not only to reduce the design time considerably, but also to improve substantially the mutual cohesion of the tender documents. The first drafts were committed to paper in February 2011. Construction started in May 2012, and the building was already in use by June 2013.
Dr Vermes says: ‘I have never participated in such a smooth process, with so much spirit and enthusiasm. This is an exemplary project that can be considered best practice in cleanroom construction.’
| Project spec | |
| Client | Erasmus MC |
| Architect | EGM architecten |
| Mechanical & electrical installations engineer | Royal HaskoningDHV |
| Construction engineer | Aronsohn raadgevende ingenieurs |
| Building contractor | Strukton Bouw & Onderhoud |
| General contractor for cleanrooms, mechanical, electrical clean utilities and validation | Kropman bv installatietechniek |
| Cleanroom contractor | KCS Cleanroom systems |
| Contract/site management | BOB Advies |
| GMP consultant | Pharmatech consultancy |
| Fire safety, construction physics consultant, site supervisors | EGM adviseurs |
| Building size | 4,300m2 GFA |
| Design period | Feb – Sep 2011 |
| Construction preparation | Sep 2011 – May 2012 |
| Implementation | May 2012 – June 2013 |
| GMP classification and air pressure hierarchy | The production facility roughly comprises three zones – an aseptic compounding section (GMP classes C and D); a non-sterile section (GMP class D); and a sterile section (GMP classes B and C). In the framework of manageability, the smallest pressure step is 7.5 Pa. The hazardous rooms are equipped with an airlock. |




