VitaSquare Wolvega produces nutritional premixes. In the Netherlands, the company enriches dry or ‘instant’ food with vitamins, amino acids, minerals, trace elements, and many more components.
For this to work, the nutrients need to be added in powdered form.
For the design of the specific spray dry plant for small scale production (≤ 1 MT/yr), many design considerations are implemented.
First of all process steps are identified and assessed for: product susceptibility, the risk of spilling, the method to clean in place as well as the exterior, including the room. Logistics of materials, product, staff and waste are also planned for.
Dosing and fine ingredients mixing
Logistics are set up to handle most raw material in bulk containers, positioned outside the process rooms, are hooked up to the process lines (Image 1). This all is done in a non-classified but controlled area of basic hygiene level.

Image 1
Spilling is not expected as all raw materials here are in containers or per piping such as feed water. Also, the CIP and waste flows are run as fixed piping. Specific additives are added in the processing room via a tank lid. (Image 2)
The tank itself is at the other side of the wall, with all its pumps, valves, sensors, actuators, wiring and supports. This leaves the processing room quite easy to clean. The various connections for the process and transfer steps can be made by transfer panels. The fine ingredients come to this processing room by an elevator from the ground floor. The elevator acts as an airlock as it is interlocked and flushed by overflow air maintaining the pressure flow cascade.
As shown in images on p55, the logistics are well separated as well as the majority of the process equipment is located in a non-classified hygienic zone. The area of exposure is very limited and in a controlled; ISO Class 9 environment.
Where the product is transported and processed further in closed equipment basic hygiene is maintained although not particularly necessary unless a breakdown or maintenance situation occur: All fluid piping is designed and equipped to be clean in place (CIP) treated. Plus, a specific part of the facility is designed around the spray dry tower and equipment.
Unique design features are:
- Clean corridor concept in combination with a pressure flow cascade design: This implies the corridors surrounding the process equipment areas be cleaner and better controlled than the process area. The benefit of this is twofold: a) any product residues spreading out of the equipment is prevented to spread around and b) only clean supply ducting outside the process is needed.
- Box in Box design: The influence of any pressure/flow dynamic from wind attack is taken away by a surrounding extra envelope. This also allows visitors to gain visual access to the plant without gowning up and entering.
- Gravity settling of product spill: The extract air is returned via a wide shaft with a very low air velocity upwards to the air handling system. This will allow any relative large spillage particles to settle at the bottom. Only airborne particles will be carried up and away.
- For areas that require wet cleaning a specific exhaust is opened when cleaning and drying to avoid undesirable damp air recirculating.
Specific data of the system:
- Supply air filtering: F7, F9 (EN779) and finalbfiltering E11 (EN1822)
- Classification by particles in air in operation ISO 8 at 0.5 ≥µm
- Process air E12 (EN 1822) to the spray dryer at ISO 7 at 0.5≥µm
- Passive overflow cascade design with nominal highest pressure of 40 Pa
- Twice or trice reused all overflow air in clean corridor concept reducing the required total air volume by a factor of 2.5
Differences in approach
Hygienic design of food processing plants is not specified in required cleanliness levels unlike sterile pharma products in EU GMP Annex 1.
In the EHEDG DOC 47 (sept 2016): ‘Guidelines on air handling systems in the food industry’, information is given on how an air handling system should be designed and constructed. For those areas of High hygiene, no direct criteria for levels of contaminants are given. For the design only the essentials to be considered and the recommended filtration steps are given as shown in Figure 2.
In the section on Air Quality monitoring (section 7.5) the recommendation is given: “Air quality monitoring in a food manufacturing environment should be implemented to control dust and microbiological contamination risks caused by people, the processes and the environment.... Air quality monitoring may include:.... Airborne particle concentration monitoring…. Microbiological monitoring based on zoning hazard analysis, optional for zone B, recommended for zone M and essential for zone H”.
Comparing the above to the EU-GMP Annex 1, it is clear the food industry has a challenge in establishing and demonstrating control on the safety of their products.
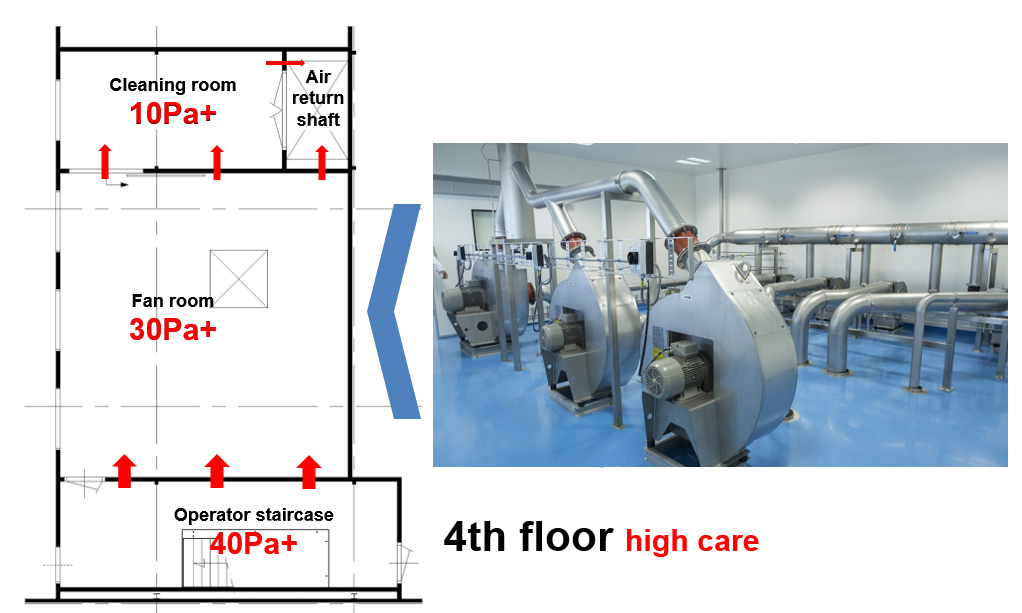
Figure 1A: The fan area for the spray dryer inlet on the 4th floor
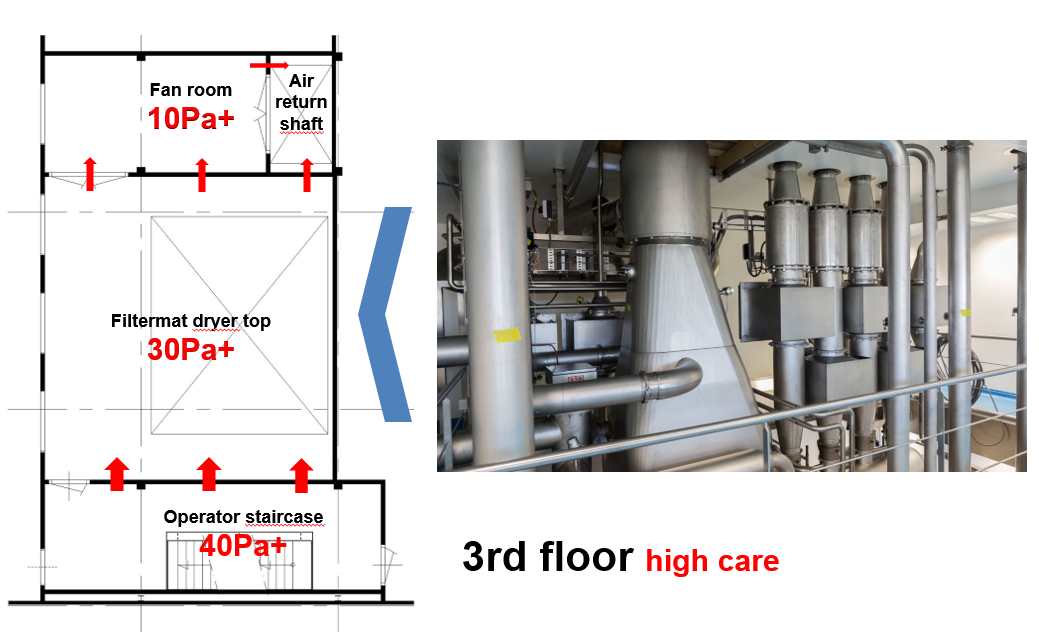
Figure 1B: The fan area for the spray dryer inlet on the 3rd floor
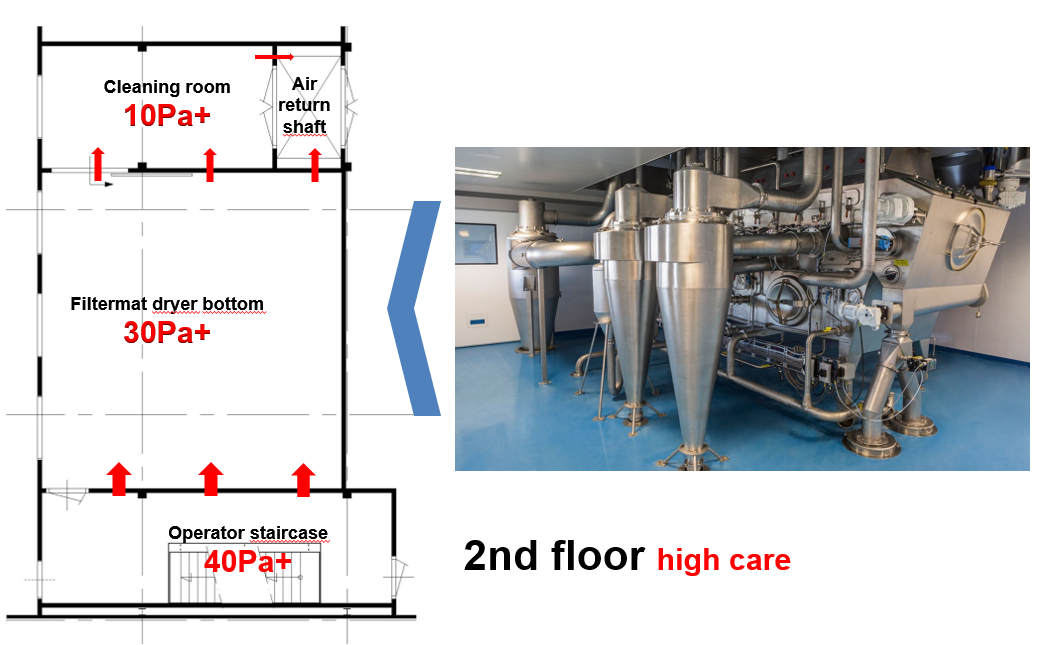
Figure 1C: The spry dryer filtermat bottom at the 2nd floor
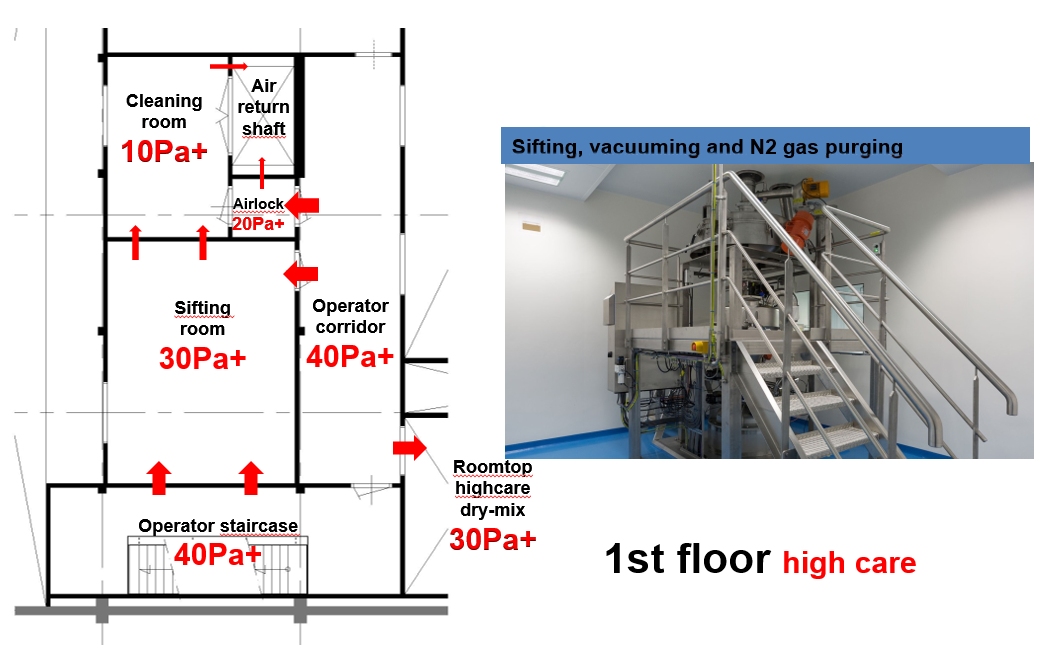
Figure 1D: The sieve for the spray-dryed product on the 1st floor
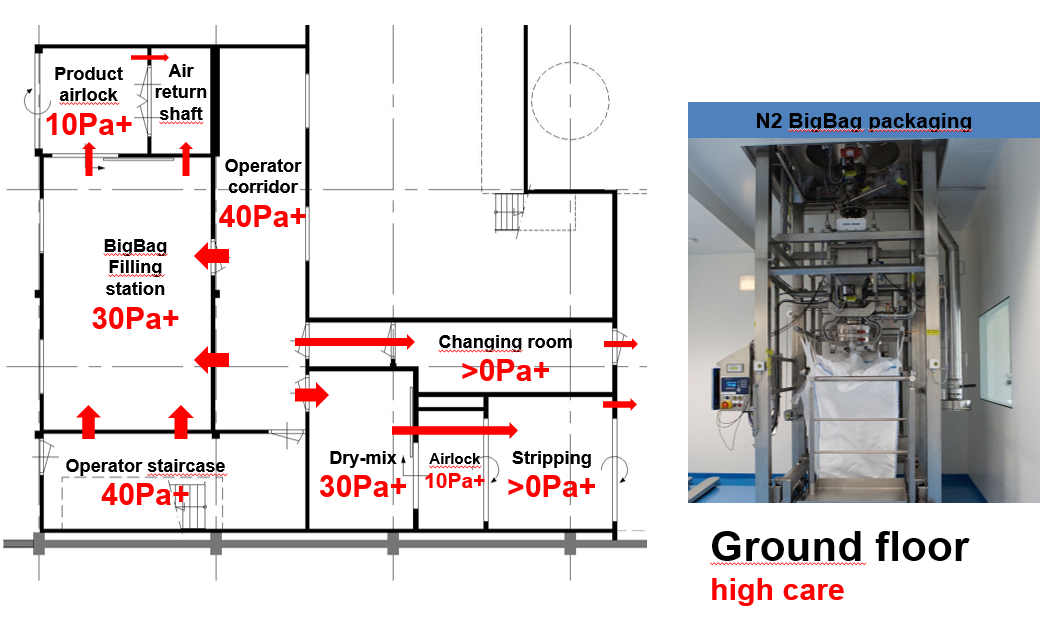
Figure 1E: Big bag filling, dry mixing, gowning and airlocks on the ground floor
Conclusion
Contamination control is essential in high hygiene production zones, but no specific contamination levels are given. Microbial (re)contamination is the most important concern, where specific pathogenic contaminants are of special focus.
The HACCP approach is essential to establish and demonstrate control. EHEDG guidelines contribute to the design for processes and their surroundings. Integrated design is mandatory and has the most optimised outcome with respect to investment, operation, and cleanability.
