Protective equipment has to fit to be effective. Jason Kelly, Kenelec Scientific, looks at a method of testing whether protective masks fit well and are working effectively when worn by the wearer.
Pharmaceutical manufacturing requires many processes and quality checks throughout the production lifecycle to verify the sterility and integrity of the product. Environmental monitoring and cleanroom conditions are employed to ensure that the product is manufactured in a contamination-free environment.
A lot of time and effort is spent within the product lifecycle to protect the product before it makes its way to the patient, which is all justifiable considering patients with low immune systems have undergone surgery and rely on safe products for their recovery. But what about the people who work in these manufacturing environments – are they protected from the product with the same rigour?
Many pharmaceutical products could affect the health of the people working in pharmaceutical cleanrooms should they accidentally be exposed to them. So does industry do enough to limit the exposure of these harmful products to the personnel manufacturing them?
First, cleanroom personnel wear special protective cleanroom clothing. They gown up before entering the cleanroom, donning garments that cover the full body, such as Gore-Tex body suits, hoods, booties, latex gloves, face masks and safety glasses. But the emphasis of this type of protective clothing is often to protect the product from the people working in the processing environment rather than the other way round.
Certain products – cytotoxic injectables, tablets and nano-based pharmaceuticals, for example – require extra steps, such as Restricted Access Barrier Systems (RABS), Laminar Air Flow (LAF) and Bio-Safety Cabinets, to protect the operators from exposure. The recent development of nanotechnology provides an added unknown risk as the potential harmful effects of nanoparticles are still being researched, but some results obtained so far have raised international concern.
In traditional pharma manufacturing, glass vials regularly break during processing and product is splashed and propelled into the environment, while tablet processing generates dust that consists entirely of product. So the processing environment can be a potentially dangerous place for cleanroom personnel working there on a daily basis.
All too often, at some point, the operator is in direct contact with the product, either during the manufacturing process at the fill head or later on, when running routine quality checks on the product.
Many pharmaceutical companies routinely carry out health checks and blood tests on employees working in these environments to pick up any anomalies.
Need for monitoring
Likewise, the wearing of cleanroom clothing and Respiratory Protective Equipment (RPE) is not enough on its own: there needs to be some monitoring or checks in place to test the integrity of the RPE.
Legislation provides instructions for employers on how to protect employees from the work environment. In Australia, the recently updated ASNZ-1715 standard for RPE (Face Masks) requires mandatory “Fit Testing” of all employees wearing RPE. Now employers in all industries are required to Fit Test their employees.
Fit Testing ensures the wearer of RPE chooses the right size mask and wears the mask correctly. In the pharmaceutical industry, employees wearing face masks must be tested at least annually.
A proper Fit Test verifies that the mask is correctly sealed around the wearer’s face. The test is of the quantitative type and is based on a ratio of ambient air particles versus particle count inside the mask. The seal is tested and the higher the ratio, the better the seal. It is the most accurate type of test available.
In reality all face masks leak for various reasons – some masks are cheaply manufactured and some models are not suitable for the wearer’s face as we all have uniquely different size faces and no one mask fits all. The wearer’s face can also change over time with weight gain or loss or if facial hair is present or not.
Performing the test
A specialised particle counter is used to test the fit of a particular type of mask. One manufacturer, TSI, leads the way worldwide with the PortaCount fit testing system, which uses light scattering techniques and condensation particle counter technology (CPC) to detect the particles in the ambient air and inside the mask while the mask is worn. The test is carried out while the user wears the mask to verify that an appropriate fit factor (seal) is obtained by the user.
| Fit factor pass/fail levels | |
| Breathing apparatus | |
| Full face | |
| Half face | |
| P3 disposables | |
| P2/P1 | |
The test protocols are outlined in different standards around the world. Occupational Safety and Health Administration (OSHA) in the US leads the way and is probably the most common standard adopted.
The PortaCount measures the upstream particle concentration (ambient air) at a designated size and the downstream particle concentration found inside the mask. The ratio of the two concentrations is called the “fit factor” and the higher the fit factor the better the seal between the mask and the user’s face (see fit factor pass /fail rates given in the panel below).
Because the size of the test particles are in the submicron range, the PortaCount must grow the particles inside a chamber prior to detection. It does this using CPC technology, where alcohol condenses on and around the particles so their diameter is large enough to scatter light and the photo detector can detect the scattered light and count the particle.
When disposable P1 and P2 type masks are tested, the PortaCount must select a size of particle that can be tested, as the efficiency of disposable masks is not as efficient as full- and half-face masks.
In this case, the unit can internally select the particle size used during the test by pulling the particle from the poly-dispersed particle stream using a Dynamic Mobility Analyser (DMA). The DMA negatively charges all the particles passing through the unit and selects a particular size by pulling only particles with a particular charge from the main poly-disperse stream. It then employs these particles to challenge the mask.
If the DMA were not used then the test would always fail, as P1 and P2 mask efficiency is so low that small particles present in ambient air freely pass through the mask. For this reason the DMA selects a larger size particle to use when conducting the tests.
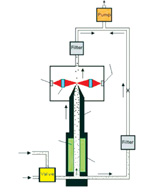
Schematic showing the operation of the PortaCount
Many other industry sectors, such as mining, the fire service, hospitals, police forces, governmental departments and the army, routinely run employee monitoring programmes, some of which have been running for several years. It is surprising, then, that the most highly regulated industry is dragging its feet and being slow to adopt this mandatory testing using this proven technology.
Setting up such an employee fit testing programme is not as complicated as might be expected. The new PortaCount 8038 with user inter-face touch screen and pass/fail results, database storage and standalone or PC connectivity modes, allows fit testing programmes to be adopted with greater ease and instantaneously.
Employee safety should be the first goal next to product quality and integrity. Fit testing not only verifies that the user wears the correct size mask but also trains the user on how best to fit the mask to their face to get a good seal. It is this good seal that prevents the user from breathing in harmful pharmaceutical products.




