The process of controlling contamination in pharmacy and medical device manufacturing should be preceded by the performance of a risk analysis and validation so that the organisation can effectively implement the contamination control plan. One of the elements of the contamination control plan is the selection and validation of the use of a sanitisation system that will effectively and efficiently contribute to the elimination and prevention of dust and microbial contamination.
Specialised cleaners used to sanitise and disinfect work areas where the drug and medical device manufacturing process takes place are an area of particular focus.
This publication will outline the key aspects involved in the approach to developing an organisation’s contamination control plan and the selection and validation of specialised cleaning products based on Evolon technology, in the context of an ISO5/AwB cleanliness class application for an aseptic process.
- Contamination control plan
The requirements of ISO standards and GMP legislation for sterile production are very stringent. The manufacturer of a drug or medical device is required to carry out supervision and continuous control of environmental parameters so as to ensure the sterility of the technological process and, therefore, the safety of the final product.
Based on legal requirements, the manufacturer is required to establish, implement and maintain a formal system called a contamination control plan. This system is based on several main elements:
- Determination and identification of potential sources and routes of contamination in clean rooms
- Risk assessment of identified pollution sources and routes and their reduction with the implementation of appropriate and proven pollution control methods
- Develop a monitoring plan with appropriate sampling methods to control sources of pollution and implement surveillance methods
- Determination of acceptable, alarm and action limits for dust and microbiological cleanliness
- Verification of programme effectiveness
- Development and maintenance of system documentation
- Staff education and training
The basis for developing an effective contamination control plan for the manufacturing process of sterile medicinal products and medical devices is a risk assessment, which is also the prelude to the validation process, including the validation of the sanitisation system.
Risk assessment is a fundamental part of an organisation’s risk management. It allows the identification, assessment, reduction or elimination of contamination risks, which affects product quality and ultimately patient and consumer satisfaction.
The risk assessment should be monitored, should identify the variability in the manufacturing environment, including the cleanroom environment, and should take into account the type of contamination that should be subject to surveillance.
In the areas of pharmacy and medical devices, risk assessment is usually carried out using the FMEA method.
- Specialised cleaners for the sterile processing of medicinal products and medical devices
GMP and the ISO 13485 standard require the manufacturer to identify an effective method of eliminating contamination and supervising the manufacturing environment. In developing a contamination control plan, the first step is to identify possible sources of dust and microbial contamination for which, following a risk analysis, it will be possible to select an effective control system, including a sanitisation system.
The risk assessment for the sterile drug and medical device manufacturing area should consider several key sources of particles and microbial contamination, which, introduced with personnel, starting materials and equipment, are a key element in the transmission of contamination into the manufacturing area.
Key areas to be reviewed include:
- Clothing - Elements, material, design, comfort, utility, frequency of dressing, choice of laundry, transportation
- Personnel - Selection of personnel, competence, safety, maximum number, transferability, contamination, morbidity, movement, entry, exit
- Equipment - entry, exit, installation, pollution generation, heat, humidity, maintenance and repair, replacement equipment, maintenance of cleanliness
- Materials - selection, entering, cleanroom storage, electrostatics, waste cleanliness, packaging
- Cleaning maintenance - Personnel, material entry, sanitisation methods, breakdowns and scheduled downtime, monitoring frequency
A key element in the selection of a sanitisation system for high ISO and GMP purity classes is the sterility aspect. When offering a sterile sanitisation system, the solution provider should ensure that sterility validation has been performed and provide the necessary certificates, as well as ensuring that the materials are triple-packed so that the system can be transported safely to purity class A/B.
Nevertheless, an important aspect to consider in the material selection process is its susceptibility to particle release into the environment. Due to the stringent requirements of both GMP and ISO 14644 in this regard, only dust-free materials can be accepted into the sanitisation system validation process. It is advisable that the dust-free nature of the materials is confirmed by appropriate certificates, such as the Fraunhofer Institute IPA certificate.
- State-of-the-art technology for the safe manufacture of medicinal products
Companies producing specialised materials and sanitising solutions for cleanrooms are constantly developing new technologies to ensure maximum protection of the working environment as well as process and product safety.
These cleaners are characterised by dust-free, highly effective removal of biological contaminants from surfaces, as well as resistance to mechanical damage and the possibility of repeated sterilisation.
In addition, specialised cleaners offer the ability to remove contaminants, including liquids, from surfaces without leaving a residue (so-called LOW RESIDUE).
- Requirements for specialised cleaning cloths in the area of high ISO and GMP cleanliness classes.
Speciality cleaning cloths enable work surfaces to be effectively disinfected, thus maintaining the dust and microbiological quality of the area.
The purpose of the cleaner is to mechanically remove contaminants from the surface with the greatest possible efficiency. There are various solutions on the market, depending on the structure of the material, the cleaning properties, the effectiveness in removing bacteria and viruses and the degree of dustiness.
In the case of high ISO or GMP cleanliness classes, the materials from which the cleaning cloths are constructed should meet several basic conditions:
- The material should be dust-free - that is, it should not be a source of particles that would be a hazard in the production process.
- Where possible, the material should be constructed in such a way that it effectively removes contaminants visible to the naked eye without damaging, abrading or disturbing the work surface
- The structure of the material should ensure maximum efficiency in removing microbiological contaminants, especially for cleanliness classes A/B GMP
- If the cleaning cloth is used in a sanitisation process, the recommended performance parameter is adequate absorbtivity - which contributes to the effective use of disinfectants on the cleaned surface.
- Certified for use in the highest ISO cleanliness classes
- For GMP Class A/B rooms, cleaners should be sterilised so as not to be an additional source of microbiological contamination
Specialised cleaners based on Evolon technology
Evolon is an example of dust-free materials used in cleanrooms of high ISO and GMP cleanliness classes. It is a type of advanced microfibre composed of alternating polyester (70%) and polyamide (30%) fibres. The way in which the microfibres are woven and the structure of the microfibres allows them to be 10x thicker than standard polyester fibres.
The structure of the material ensures high absorbency and dust-free and leaves no residue when removing liquids from the disinfected surface. The structure of the material provides high antimicrobial, including antifungal, protection. Tests have shown that the material can remove 99.999% of microbial contaminants in so-called 'dry' disinfection, i.e. without the use of a disinfectant. It can be assumed that the use of the material with a disinfectant liquid will contribute to even greater efficiency in removing contaminants from surfaces.
The fibre arrangement provides filtering properties retaining 100% of particles larger than 1 micron. The material is breathable - water vapour and air pass through microchannels. It is environmentally friendly. Produced naturally, it contains no glue, solvents, PVC or other chemicals. The surface of the material is laser-cut, which minimises the release of particles from the surface into the process environment.
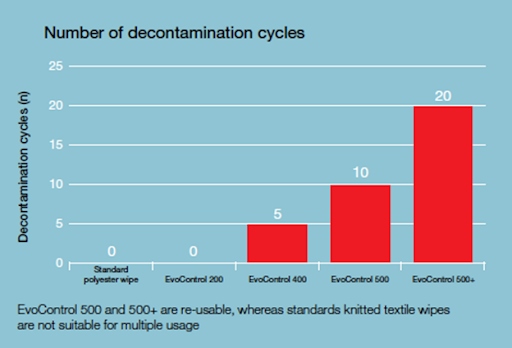
Another advantage of Evolon material is that it is reusable. This is an extremely rare feature of polyester materials, which are usually used once and then sent for disposal. The material is validated for 20 washing and sterilisation cycles which, in financial terms, makes a significant contribution to the economy of the solution.
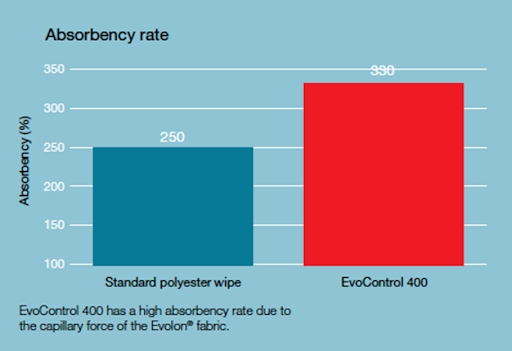
The high absorptivity of the material, in contrast to classic polyester solutions, is an undoubted advantage for disinfection of cleanroom surfaces in the area of pharmaceuticals or medical devices. While polyester does not need to be highly absorbent as a surface particle pick-up material in electronics or optics, where microbial decontamination is also important, the high absorbency of the material is of great importance. This fundamentally affects the effectiveness of sanitisation and disinfection as well as the process of leaving residues on the surface. Absorbent materials distribute the liquid with the disinfectant on the surface in a balanced manner, thus enabling the agent to maintain an adequate duration of action.
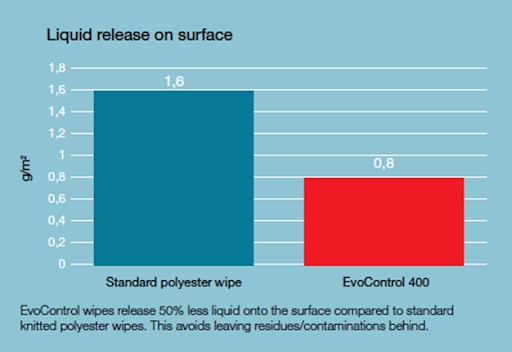
Unlike conventional polyester materials, which "poorly" hold water and as a result tend to leave much more water on the surface, Evolon, through its high absorbency, allows for a 50% reduction in the amount of water spread on the surface and thus a reduction in the amount of residue that can be a source of biological contamination. In a nutshell, the material distributes as much water as necessary to ensure the proper action of the disinfectant, but without wetting the surface too much.
Evolon material can therefore be considered to meet the most stringent requirements for specialised materials and cleaners in drug and medical device manufacturing processes at the highest ISO/GMP cleanliness classes. The incorporation of this and similar solutions allows for the effective implementation of the contamination control plan in the organisation and thus the safety of the manufacturing environment and the product itself.




