The International Society of Pharmaceutical Engineers (ISPE) has published the second edition of GAMP5; Good Automated Manufacturing Practices this August 2022. According to International Task Force, a special IPSE SME group, the rationale for GAMP5 Second Edition was “aimed at protecting patient safety, product quality, and data integrity by facilitating and encouraging the achievement of computerised systems that are effective, reliable, and of high quality.”
GAMP provides practical guidance that facilitates the interpretation of regulatory requirements, establishes a common language and terminology, promotes a system life cycle approach based on good practice, and clarifies roles and responsibilities. It is not a prescriptive method or a standard, but rather provides pragmatic guidance, techniques, and tools for the practitioner. This guide offers a robust, cost-effective system when applied with expertise and good judgement. ISPE GAMP5 Guideline comprises a main body and a set of supporting appendices. The main body provides principles and a life cycle framework applicable to GxP regulated computerised systems. Practical guidance on a wide range of topics is provided in the supporting appendices.
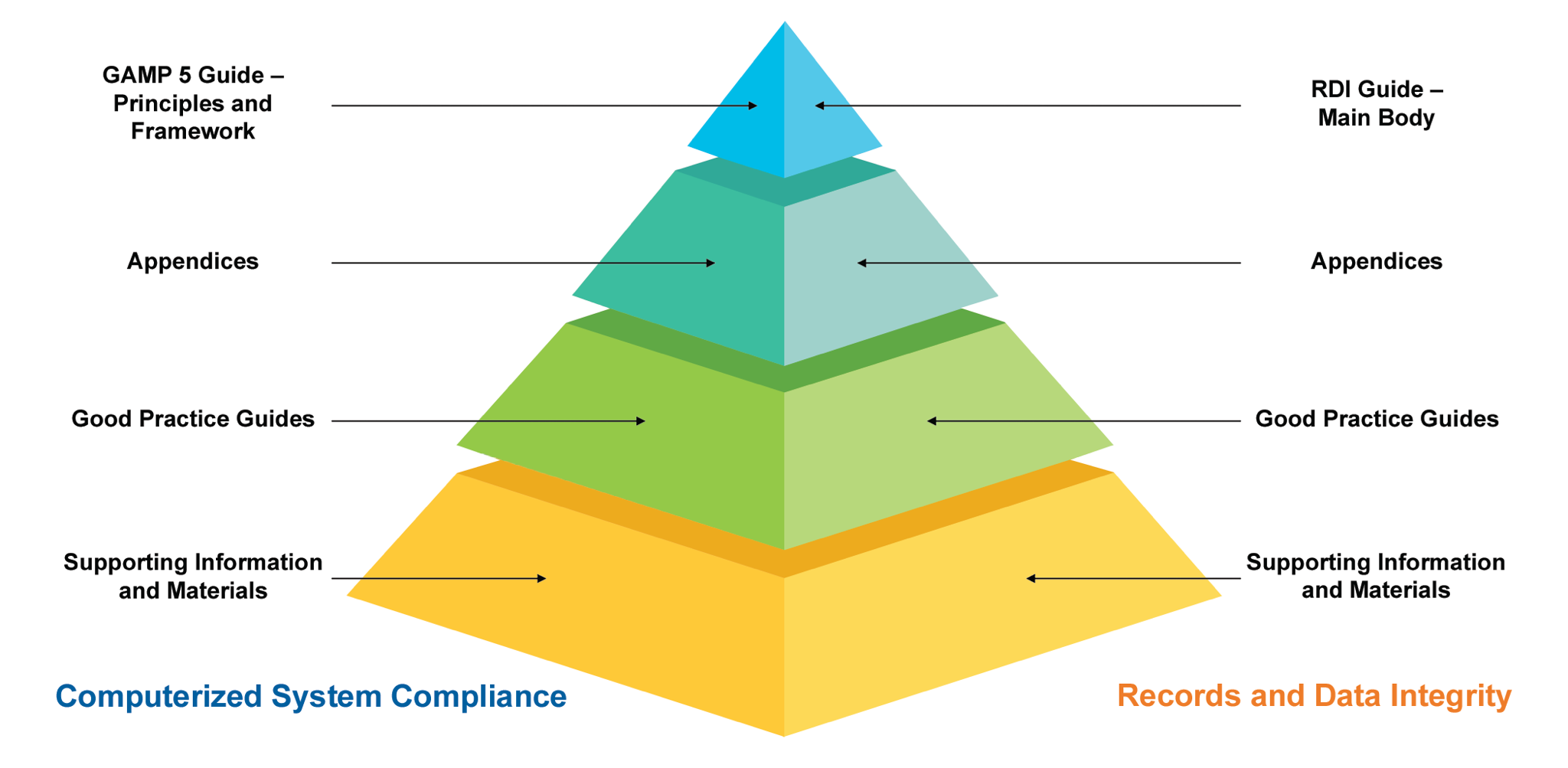
Figure 1: Overview of GAMP Documentation Structure
Apart from the main body of the GAMP5, there are appendices to enhance the subject and for a better explanation with examples, documents, and references. There are five main appendices, including:
- Management Appendices (M)
- Development Appendices (D)
- Operation Appendices (O)
- Special Interest Topics Appendices (S)
- General Appendices (G)
New and revised materials in ISPE GAMP 5 Second Edition
There are new appendices including “Agile (D8), Software Tools (D9), Distributed Ledger Systems known as “Blockchain Technologies” (D10), IT Infrastructure (M11) and Critical Thinking (M12)”. Appendices such as “Specifying Requirements” (D1) and “Electronic Production Records” (S2) have been updated significantly while some other appendices were removed (Appendix D2 – Functional Specifications, O7 – Repair Activity and S5 – Managing Quality within an Outsourced IS/IT Environment) as a result of these new additions and the revisions. As per the Task Force definition: “The overall GAMP 5 framework, key concepts, system life cycle, specification and verification approach, and Quality Risk Management (QRM) process (aligned with ICH Q9 [14]) remains unchanged.”
A synergy with the ISPE initiatives
Over the next couple of years, there are important topic areas that have been developed and maintained by the ISPE such as “Knowledge Management”, “Advancing Pharmaceutical Quality”, and “Pharma 4.0”. Thanks to the new appendices and revised main body, the new edition of the GAMP5 is now synchronised and synergised with this topic area. With the ISPE definitions, a short description of these topic areas will help us better understand the second Edition of the GAMP5.
Firstly, focusing on how organisations create, manage, and use knowledge throughout the life cycle of a product, enabling organisations to better manage their knowledge as a key asset, in turn improving the effectiveness of the pharmaceutical quality system, and providing operational benefits.
Secondly, building industry-for-industry tools and programs to help companies assess and improve their quality operations.
Thirdly, providing guidance, aligned with the regulatory requirements specific to the pharmaceutical industry, to accelerate Pharma 4.0 transformations. Also known as the Smart Factory, the objective of Pharma 4.0 is to enable organisations involved in the product life cycle to leverage the full potential of digitalisation to provide faster innovations for the benefit of patients.
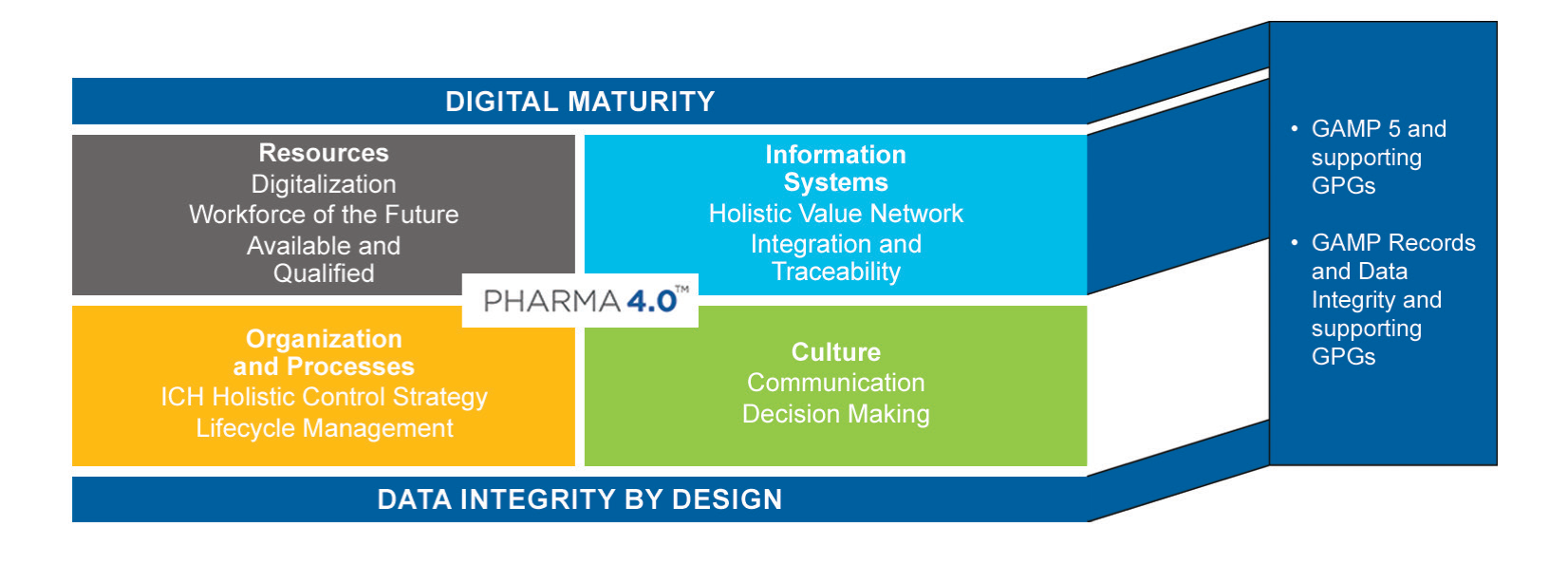
Figure 2: Pharma 4.0 operating model
Key concepts of the ISPE GAMP5 Second Edition
There are five key concepts in the ISPE GAMP5 that is applied through the process:
- Product and process understanding
- Life cycle approach within a QMS
- Scalable life cycle activities
- Science-based QRM
- Leveraging supplier involvement
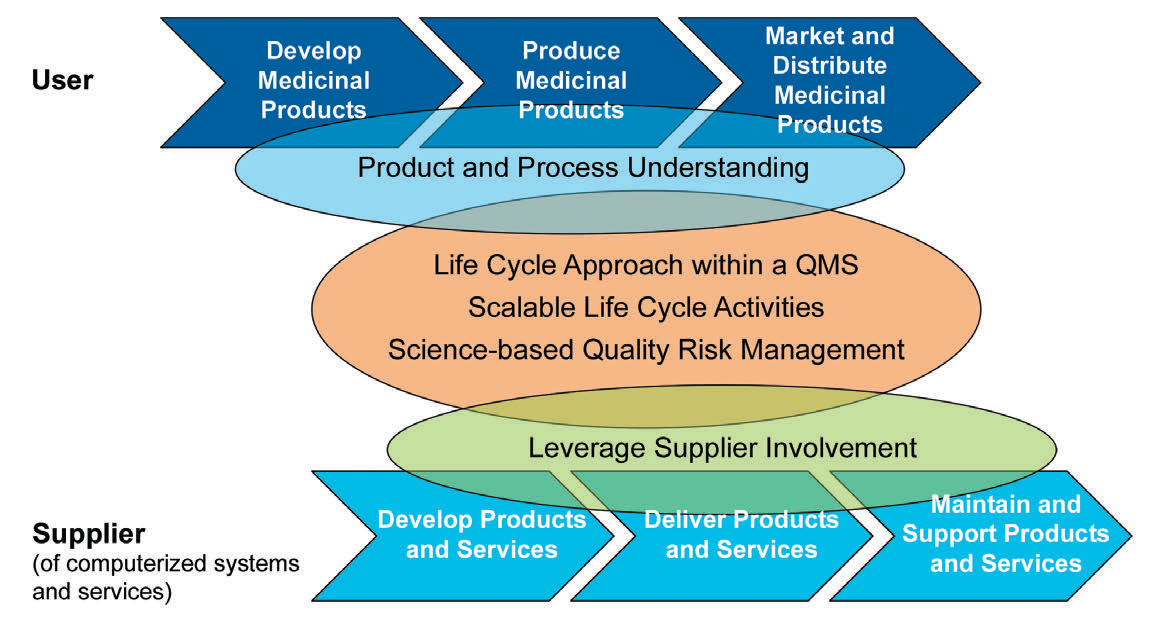
Figure 3: The five key concepts of GAMP5
Without a proper understanding of the process, any application, including the GAMP5 environment will not be possible. Thus, the multi-disciplinary involvement of different departments such as engineering, production, planning, quality, etc., is critical to make science and risk-based decisions. Another critical point here is to understand that the process is a never-ending cycle unless you decide to retire, destruct or mitigate the system. For that reason, the life cycle approach is critical.
There should be periodic reviews of the plans to ensure that the changes in this living system are defined and well-addressed. In most cases, the supplier has the most critical information that should be transferred or exchanged with the stakeholders.
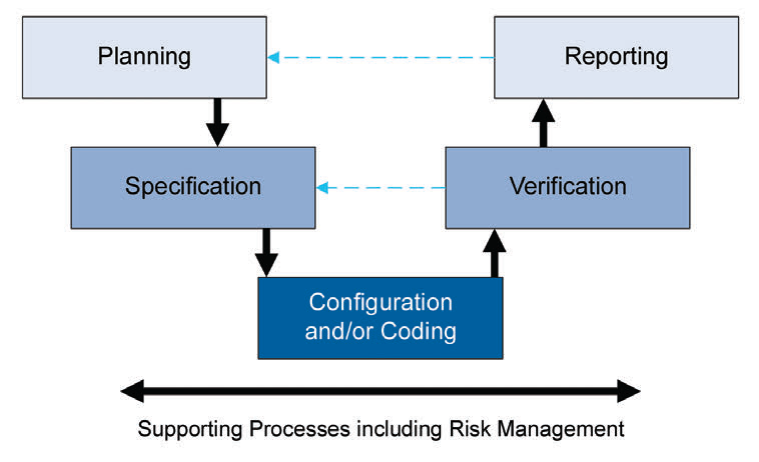
Figure 4: Process activities for risk management
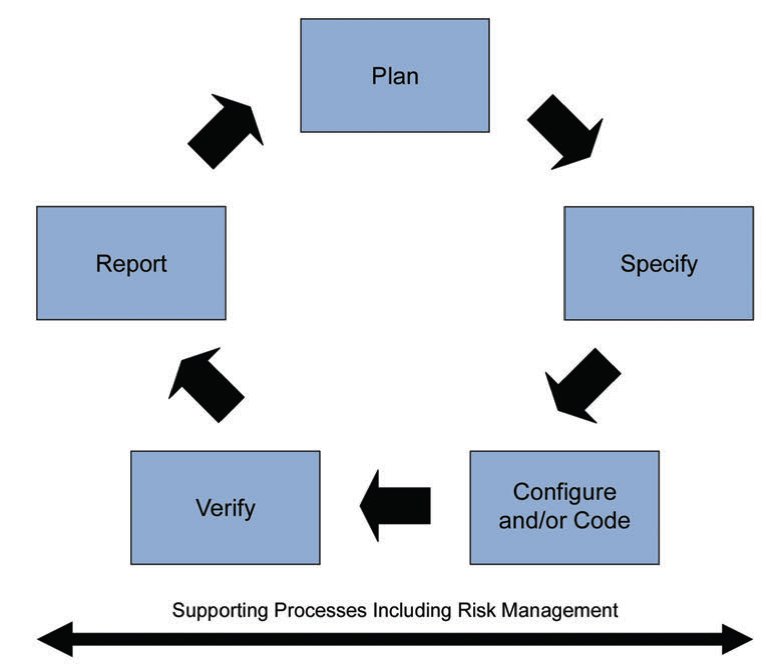
Figure 5: Iterative and Incremental Approach for achieving compliance and fitness for intended use
Leveraging this supplier involvement will allow you to get a better understanding, and easy to adopt and integrate systems and maintain them seamlessly thanks to this know-how from the supplier. As part of this “life-cycle approach”, the second edition is now covering “Iterative and Incremental Approach” for achieving compliance and fitness for intended use. Classic linear approach was suggesting a one way development, starting with planning and specification to the verification and reporting as a final step after successful configuration and/or coding.
New iterative and incremental approach has a life-cycle structure that will allow us to review, update and re-visit steps to achieve compliance and fitness for intended use.
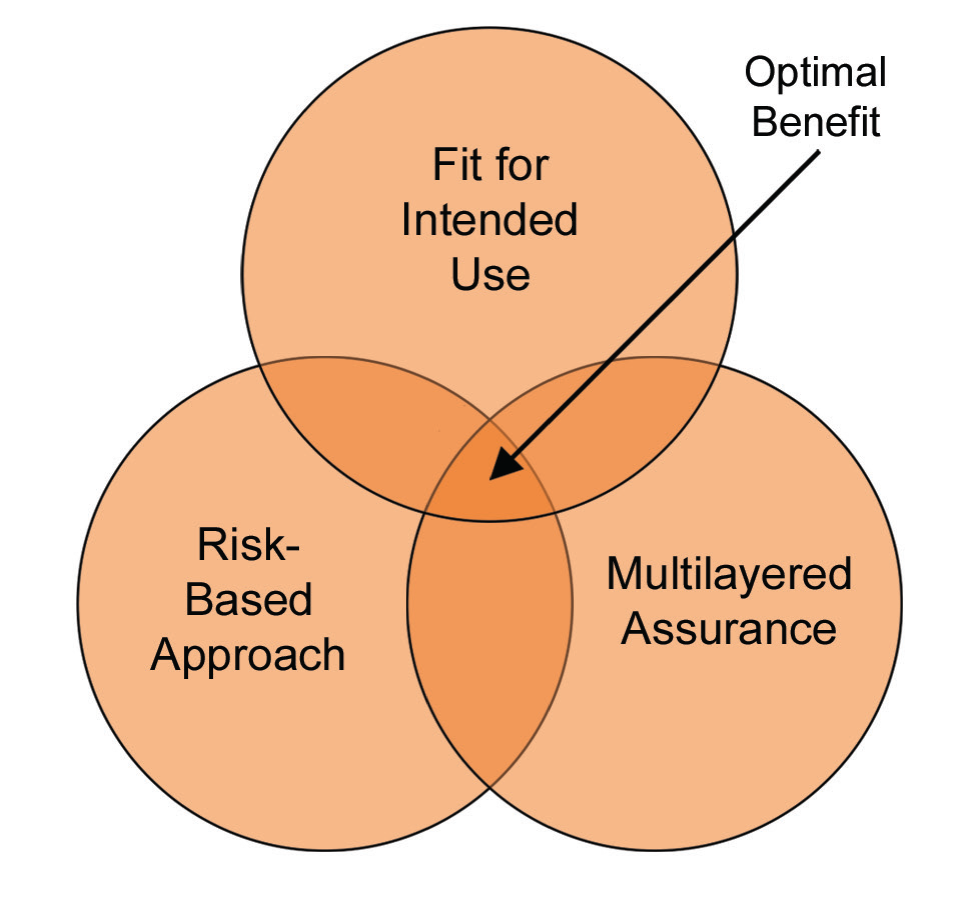
Figure 6: Critical thinking changes in the approach to risk
Maybe critical thinking is the most important change in this second edition. We have been using the “risk-based approach” for a while, however over-thinking or over-estimating due to an excessive amount of “risk assumption” sometimes put us in the worst situation than where we initially started. Of course, we will continue implementing the risk-based approach however, as shown in Figure 6, we will focus on “optimal benefit” thanks to the critical thinking approach.
ISPE GAMP5 Second Edition can be found in ispe.org website, under “Publications, Guide Documents’. All image copyrights are ISPE 2022.

