Due to the numerous benefits of presaturated wipes — ease-of-use, lower VOCs, increased convenience and reduced hazardous waste — their use in the pharmaceutical and healthcare sectors has proliferated over the past few years. The original presaturated wipes were, in the main, alcohol-based while disinfectant presaturated wipes were less widely used.
There are now numerous disinfectant wipes available, used in both cleanroom and healthcare environments. With the changes to the transfer disinfection requirements for specials manufacturers in the UK and unlicensed aseptic units, now including a sporicidal wiping phase, there are also more sporicidal wipes available.1,2
However, as discussed in a previous article3, as there are known problems with adding certain disinfectant active ingredients to certain wipe or mop substrates,4,5,6,7 how does a customer ensure that a presaturated wipe works as effectively as a ready-to-use (RTU) fluid?
Disinfectant/substrate incompatibility
When previous testing work was carried out there was no specific European test for presaturated wipes. Contec used the existing standard EN suspension and surface tests on eluate, extracted from the presaturated wipes at the end of their shelf life, to show that the wipe being in contact with the fluid did not have an adverse effect on the wipe. Work was carried out on a range of sterile and non-sterile presaturated wipes currently on the market.8
Wipes at various points within their shelf life were squeezed in a standardised method to remove as much fluid as possible from the wipe and the eluate was tested to standard EN disinfectant methods: EN 1276 for bacterial efficacy, EN1650 for fungal efficacy and EN 13704 for sporicidal efficacy.9, 10, 11 Table 1 shows the results obtained and where the wipe eluate did not reach the level of efficacy as claimed for the equivalent fluid product.
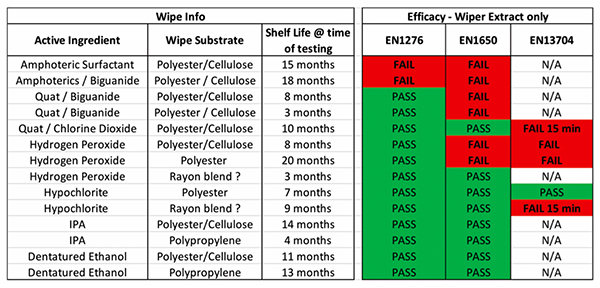
Table 1
The contact time to prove sporicidal efficacy against EN 13704 is 60 min. However, as this tends to be impractical in use, many companies test at a shorter contact time of between 3–15 min. The test work was carried out at 15 min — a longer contact time than claimed for any of the products tested. As the results show, many of the wipes did not meet the claims made for the equivalent RTU fluid. The tests were performed twice to eliminate any possible testing error (all tests did pass validation).
It was concluded that the wipes had probably failed for a number of reasons. Hydrogen peroxide (H2O2) on contact with certain materials is likely to start the decomposition process, known as “gassing off”; it breaks down to water and oxygen, which can leave a wipe with little to no activity and a pouch overfilled with air (oxygen). Additional testing showed that amphoteric and quaternary ammonium-based wipes had a significant portion of active ingredient binding to the wipe and were not laid down onto the surface.
A specific test for wipes
It was apparent that with the increase in availability and use of presaturated wipes there was a need for an EN procedure specifically for testing wipes. The current methods for testing disinfectants, unless carried out on the eluate as above, cannot be suitably modified to test wipes. The closest surface test, BS EN 13697:201512, for bacterial and fungal testing, does not include any mechanical action. There are two international test norms designed to test presaturated wipes. ASTM E2362 – 15 Standard Practice for Evaluation of Pre-saturated or Impregnated Towelettes for Hard Surface Disinfection, which is used to substantiate disinfectant wipe claims by the EPA in which contaminated test surfaces are wiped with the presaturated wipe. Either 10 or 60 contaminated test surfaces are treated with the wipe. After incubation, the number of test tubes showing growth of the target microorganism is recorded. To pass a 60 carrier test, at least 59 of 60 surfaces wiped with the towelettes must demonstrate complete disinfection (no detectable growth of the target microorganism in the tubes containing neutralising growth medium). To pass a 10 carrier test, complete disinfection must take place on all wiped test surfaces.
This test still has some weaknesses; similarly, to the AOAC spray test there is variability on the basis of statistics alone, certain test parameters are not standardised (e.g. humidity levels during drying, concentration of bacteria on the test surface). While the test includes mechanical action, which is closer to in-use conditions, wiping numerous surfaces with one wipe until all the disinfectant is used up does not mimic the wiping process in a cleanroom environment. Furthermore, the test does not obviate variability in the pressure and technique used to wipe the test surface.
A more recently introduced presaturated wipe test, ASTM E2967-15 – Standard Test Method for Assessing the Ability of Pre-wetted Towelettes to Remove and Transfer Bacterial Contamination on Hard, Non-Porous Environmental Surfaces using the Wiperator, attempts to remove some of this variability by using a reproducible mechanical action for wiping generated by the “Wiperator”.
Developed by Filtaflex and the Centre for Research on Environmental Microbiology of Ottawa University, the Wiperator is claimed to improve consistency when compared to manual test methods by accurately controlling the applied force, rubbing speed and duration of wiping. During a test, the wipe moves in an orbit of 10 mm diameter at 1 rev/sec, also oscillating through 6 degrees of arc. Wiping continues for multiples of 5 sec, up to 45 sec. The default wipe-sample contact force is 150 g, which equates to a rubbing force of 800 g or more in a real-life wiping situation (an accessory 150 g weight permits testing 300 g forces). It is open to debate whether in a cleanroom this mimics both the force and way in which a wipe is used.
Work carried out by Sattar et al evaluated the new ASTM test to demonstrate efficacy of disinfectant and detergent wipes.12 Work was carried out by three independent labs on five types of commercially available presaturated wipes. All five wipes (four detergent and one disinfectant) achieved greater than log 4 reduction against bacteria but only the disinfectant wipe prevented the transfer onto another surface. They concluded that this standard method ensured greater precision and reproducibility when testing presaturated wipes with mechanical action. However, the test is still not commonly used in Europe for testing cleanroom presaturated wipes.

EN16615:2015
Until recently there was no EN standard for assessing disinfectant wipe efficacy but EN1661513 was launched in 2015 as a carrier test for establishing whether a wipe has a bactericidal or yeasticidal effect. It is designed for presaturated wipes but the wipe could be saturated with the chemical under test at point-of-use. There are options to include an interfering substance to replicate clean and dirty conditions. The contact time can also be varied beween 1–60 min. Wiping is carried out with the wipe wrapped around a granite block weighing 2.3–2.5 kg to standardise the wiping procedure and to simulate the average pressure when wiping.
The block is wiped over four fields marked on a PVC sheet. The organism under test is dried onto field 1. The granite block is then passed over all the fields and back again in one single, fluid motion taking 2 sec. In tests on field 1; a log 5 reduction needs to be achieved against bacteria or a log 4 reduction against yeasts. In fields 2–4 there needs to be an average carry-over of less than 50 cfu for each organism.
A number of UK labs have now validated this test method and more data and understanding of the test is available. The test method was introduced with the aim of closely simulating practical conditions of application, such as contact time, temperature and interfering substances, also including pre-drying specified test organisms onto a test surface and wiping the product onto the test surface with a wipe. The lab that pioneered the test method identified key factors that affect wiping efficacy, including: wipe material, wipe quality, impregnation volume, application pressure and application technique. The intention was that the method would be suitable to differentiate between wipes.
Disinfectant manufacturers were hopeful that the test method would highlight that there is no potential cross-contamination caused by using the disinfectant wipe and it would also confirm the compatibility of the wipe substrate and disinfectant active, which we have highlighted was a particular issue with previous test work.
Potential drawbacks
Initial test work generated some results that were not expected and led to a more detailed investigation. In depth discussions about the test were also had with the test house carrying out the work (MGS Laboratories, Gosport, UK). Things to be aware of when sending product for testing against EN16615 are:
- Unless the manufacturer or facility specifies the particular wipe to be used with a fluid sent for test, the wipe used will be a standard industrial wipe, which may not behave in the same way as a cleanroom wipe. The standard specifies a 55% pulp / 45% PET wipe.
- If not specified, the wipe will be wrapped in a single layer around the granite block, this may not be the way the wipe is used in your cleanroom.
- If not specified, regardless of size or substrate of the wipe, 16 ml fluid will be used to saturate the wipe. This can have a significant effect on results. Some of the initial failures seen at the lab were the result of insufficient fluid being released from the wipe, either because the wipe was inadequately saturated or the wipe did not give up the active fluid. Many wipes saturated with cleaning in mind will only release a small amount of fluid to the surface, as a wipe optimised for particle pick up is saturated just enough to break the binding layer of fluid between a particle and the surface and not so wet that it releases the particle back to the surface again. Wipes optimised for surface disinfection need to be saturated enough so they do not become unmanageable with drips but can lay down a visible film of disinfectant for the contact time required. The substrate of the wipe also has an effect on how fluid is absorbed and released to the surface. Organic materials, such as cotton and cellulose are able to absorb fluid into the hydrophilic fibre itself while manmade materials, such as polyester, adsorb liquids into the interstitial spaces between the fibres. In general, synthetic wipes (polyester and polypropylene) tend to be more sorbent as the fibre size is reduced, with microfibre products being the most sorbent option. Manmade fibres, such as polypropylene, are ideal for laying down a metered release of solvent. The test does not look at how much fluid the wipes release to the surface. This could be easily resolved by weighing the wetted wipe before and after the test.
- There is no detailed description or validation in the test method of the swabbing technique to be used for recovery of organisms from the test squares. The standard simply states that the whole surface of each test field should be rubbed with two swabs, one dry and then wet, then swabbed again with a dry swab until the test field is visibly dry. The whole procedure should take no more than 1 min per test field. This is probably the area that could lead to the largest variation between labs, were the swab recovery to be carried out differently. An option for improvement could be to validate the swab recovery phase by checking with contact plates.
Influence of mechanical action
As a wipes manufacturer, Contec was less certain when the test was announced that it would be the definitive answer to testing presaturated disinfectant wipes as its in-house testing of wipes had always indicated that significant log reductions in viable organisms could be gained simply by the mechanical action of a wipe or mop. This is especially true of microfibre fabrics, which are ideal at picking up microscopic particles. The company was so confident in this mechanical action of wipes that instead of repeating the testing on the disinfectant wipes it had previously tested using the EN16615 test, it decided to see what results could be achieve with three different wipe substrates simply saturated with purified water.

Three different substrates of cleanroom wipe were chosen, 68 gsm polyester / cellulose blend, 140 gsm knitted polyester wipe and a 120 gsm 100% polyester microfibre wipe — all of which would be commonly used in different grades of cleanroom. As the wipes are different sizes and weights they were saturated with differing amounts of water, to ensure good layout of fluid, namely 12 ml, 15 ml and 60 ml respectively.
They were then tested against the EN16615 test with the addition of Aspergillus brasiliensis (A. Brasiliensis) as a test organism. The summary results from this testing can be seen in Table 2. Two of the substrates tested passed the EN16615 test against Candida albicans and one substrate also passed against A. brasiliensis.
If the test method is capable of highlighting compatibility issues between the wipe and the disinfectant all of the test results should have been a fail, as there was no microbiological activity on the wipe. Interestingly one of the harder to kill organisms in the test passed when using the polyester / cellulose wipe.
The yeast passed with both the polyester wipe and the polyester / cellulose wipe. This clearly highlights how significant mechanical action is in the wiping process and the ability of different wipe substrates to pick up and retain particles / microorganisms.
If the results were presented in their entirety, as in table 2, then it would be clear that there is something odd with the results and a conclusion would be drawn that the “disinfectant” was not hugely efficacious and probably mechanical action was helping to achieve the results. The concern is, if the test had been carried out solely on the yeast or fungal organisms to show efficacy specifically against those organisms, maybe because a marginal fail had been achieved on a surface test (EN13697) then this would have misrepresented the efficacy of the disinfectant.
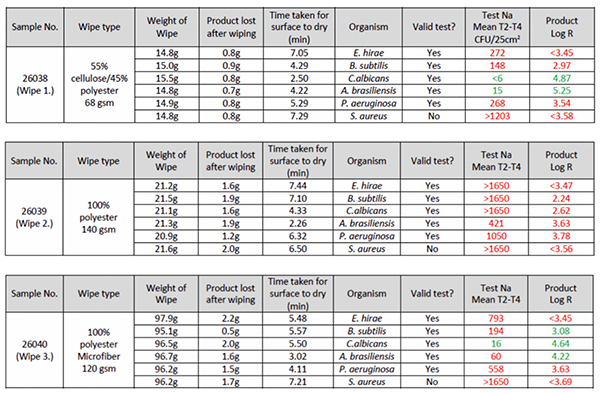
Table 2
There is an argument to say that the test validates the process, which is being carried out, the effectiveness of the fluid to kill and the substrate to pick up and retain particles. However, it is not accurate to suggest the test confirms compatibility between the wipe and the disinfectant as mechanical action could mask any potential incompatibilities.
Also, what cannot be deduced from the test is whether a wipe with no microbial efficacy does release the particles / microorgansims at some point. Great care would have to be taken to ensure wipes were only used for a validated amount of time and placed directly into waste receptacles. There could still be a possibility of organism release as a wipe was folded and refolded, for example. A simple way to check whether mechanical action is masking any incompatibility issues would be to check the wipe after testing for the amount of micobial contamination in the wipe.
Alternatively, the test should only be used as part of a panel of test work The validation of an efficacious disinfectant should include: a pass on a surface without mechanical action (EN13697); a pass using EN16615 and the cleanroom wipes to be used and, in the absence of any published phase 3 tests, ongoing environmental testing.
A presaturated wipe could be tested against EN16615 with the extra step of testing the wipe to see if microbial contamination has been killed on the wipe itself or coupled with a test of the disinfectant squeezed from the wipe and then tested against EN13697. This would confirm what is contributing to the overall result — efficacy of the disinfectant and additional reduction from good mechanical pick up from the wipe.
In summary, with the increased use of presaturated wipes in cleanrooms and new guidelines requesting use of presaturated sporicidal wipes a robust EN method for testing disinfectant presaturated wipes is definitely needed.1,2
However, due to the significant log reductions, which can be achieved with wet wiping alone, further consideration needs to be given to improving the test method and care needs to be taken when reading results in exclusion.
Acknowledgements
The author would like to acknowledge the input and work carried out by Neil Simpson, R&D, Contec and Kim Morwood, Director MGS Laboratories.
References
- MHRA Q&A for Specials manufacturers Jan 2015 update
- Guidance for Aseptic Transfer Processes in the NHS: Addressing sporicidal issues. July 2015 Edition 1. NHS Pharmaceutical Quality Assurance Committee by the NHS Pharmaceutical Micro Protocols Group. Lead authors Mark Oldcorne, Tim Sizer
- K. Rossington. (2013) Cleanroom Technology, Vol 21 (6) pp18-20
- V. Williamson. (2007) Infection Control Today, Vol 11 (8) August 2007
- R. Bloss, S. Meyer, G. Kampf.(2010) Journal of Hospital Infection, Vol 75 (11) pp56-61
- K.D. MacDougall and C. Morris. (2006) Infection Control Today, June pp62-7
- Ecolab White Paper
- Contec Tech. Note TN0515 Comparison of presaturated wipes against EN standard efficacy tests
- BS EN 1276:1997 Chemical Disinfectants and Antiseptics – Quantitative suspension test for the evaluation of bactericidal activity of chemical disinfectants and antiseptics used in food, industrial, domestic and institutional areas
- BS EN 1650:1998 Chemical Disinfectants and Antiseptics – Quantitative suspension test for the evaluation of fungicidal activity of chemical disinfectants and antiseptics used in food, industrial, domestic and institutional areas
- BS EN 13704:2002 Chemical Disinfectants and Antiseptics – Quantitative suspension test for the evaluation of sporicidal activity of chemical disinfectants used in food, industrial, domestic and institutional areas
- S.A. Sattar, C. Bradley, R. Kibbee, et al. (2015) Journal of Hospital Infection 91 319–325
- BS EN 16615:2015 Chemical disinfectants and antiseptics – Quantitative test method for the evaluation of bactericidal and yeasticidal activity on non-porous surfaces with mechanical action employing wipes in the medical area (4-field test). Test method and requirements (phase 2, step 2)





