The presence of air in an autoclave sterilisation cycle adversely affects steam penetration and contact with materials being sterilised. It is important to routinely perform an air removal verification test in the autoclave to demonstrate that entrapped air is removed and thus cannot impede the steam sterilisation process. These tests challenge the air removal performance of the autoclave’s prevacuum phase, and can also indicate leaks in the chamber and/or associated piping. It is best practice to perform daily air removal verification testing following the steriliser manufacturer’s guidelines.
The pharmaceutical, biotechnology, and medical device industries are required to meet regulatory guidelines regarding the use of air removal tests in prevacuum autoclave sterilisers. The International Organization for Standardization (ISO) along with the Association for the Advancement of Medical Instrumentation (AAMI), the United States Food and Drug Administration (FDA), British Standards Institution (BSI), and the European Committee for Standards (EN) guide the use of air removal tests. The challenge is that not all agencies are harmonised in the frequency or types of testing, and it is left to individual companies to establish the testing schedule based on risk to their specific process.
Air removal verification tests helps meet cGMPs by demonstrating the effectiveness of prevacuum cycles in steam sterilisers
Current Good Manufacturing Practices (cGMPs) require both demonstration and documentation that the sterilisation process is effective, controlled, and reproducible. Use of air removal verification tests helps meet cGMPs by demonstrating the effectiveness of prevacuum cycles in steam sterilisers. The chemical indicator sheet taken from the air removal tests provides evidence for documentation purposes.
Sterilisation overview
Saturated steam condensing onto a surface is essential for killing microorganisms that may be present on the material surface. It is important to remove air before steam sterilisation (prevacuum) and to ensure air is not being drawn into the autoclave during the prevacuum process. An integral, leak-free autoclave chamber and piping system is vital. The presence of air prevents saturated steam from contacting surfaces of the materials being sterilised, which diminishes the contact time and exposure temperature required for successful sterilisation.
In autoclaves where prevacuum pulses are employed for air removal, the air in the chamber is replaced with saturated steam through a series of alternating vacuum and steam injection pulses. This is shown at the beginning of the cycle between the times of 10 and 30 minutes, where the pressure curve is in a “sawtooth” pattern, as seen in Figure 1. 1
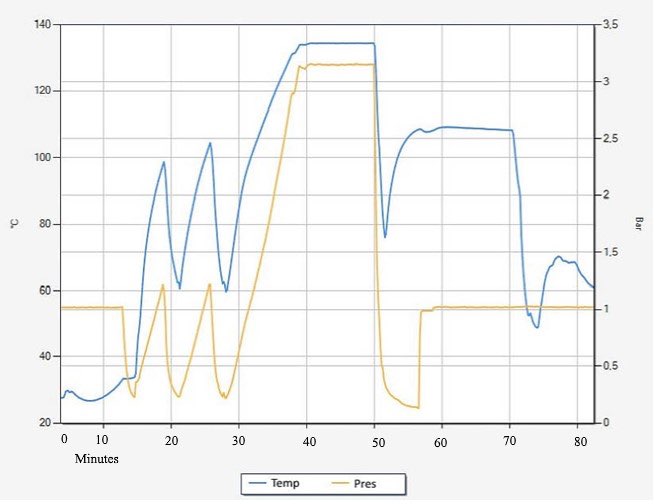
Figure 1: Simplified temperature and pressure curve for a prevacuum steriliser cycle
The number of prevacuum pulses, the prevacuum depth, and steam injection pressure must be tested and confirmed to be adequate, as these parameters have the largest impact on air removal. Other cycle parameters that affect air removal efficiency include purge and hold times at various vacuum and/or pressure setpoints.
Additionally, the load items and positioning within the autoclave chamber should be defined and tested to ensure appropriate air removal.
Air removal verification tests
Historically, air removal and steam penetration were confirmed by processing a stack of huckaback towels with chemical indicator tape inserted in the centre through an autoclave cycle. The towels presented a challenge to air removal and steam penetration. If the chemical indicator completely changed colour, this indicated complete exposure to steam and a successful air removal process.
In 1963, the Lancet journal published information on the Bowie-Dick Test, founded by Dr J. Bowie and Mr J. Dick. This test standardised and streamlined the original towel pack test2. Now, an air removal test can take one of many forms that have been demonstrated equivalent to the original towel packs, according to ANSI/AAMI/ISO 11140-43. Typically, the disposable pack consists of a small standardised test load and a chemical indicator system to detect the presence of steam. ISO 11140-4 refers to these tests as “Bowie and Dick-type tests”.
For example, a commercially available Bowie-Dick Test Pack from STERIS Corporation consists of a wrapped stack of steam penetration (air removal) barriers with a chemical indicator sheet in the centre. The test pack is placed directly in an otherwise empty steam steriliser chamber with no retaining device required.
How it works
During processing, the cycle must remove or displace the air from the barrier material and replace it with steam throughout the pack. A colour change on the indicator from yellow to a uniform blue/purple indicates adequate steam penetration, as seen in Figure 2. The thermochromic ink formulation can also assist in identifying problems with steam quality (presence of non-condensable gasses, wet steam, and/or superheated steam) and is free of lead and other heavy metals.4

Figure 2: Bowie-Dick Chemical Indicator. L-R: Unused, Pass, Typical Failure exposure
The Bowie and Dick Test Packs are pre-assembled single-use test packs designed to evaluate the performance of the air removal system of a prevacuum-equipped steriliser at 121°-124°C (250°-255°F) or 132°-135°C (270°-275°F). The test packs conform to ANSI/AAMI/ISO 11140-4:2001 and BS EN867-4 Class 2/B:2001.5
An air removal test pack is placed at the location where air is most likely to become entrained in the chamber, typically at the bottom of the autoclave and close to the drain, as shown in Figure 3. It is important that the same prevacuum parameters are used for the air removal verification cycle and the production autoclave cycle, as this is the only way the test accurately measures the performance of the autoclave air removal phase.

A Bowie-Dick Test Pack in an autoclave chamber
Regulations and standards
The following documents are relevant regulations and guidance on the use of air removal verification tests:
ANSI/AAMI/ISO 17665-1: 2006 (R) 2013. Section 12.1.6 states: “If the sterilisation process relies on the removal of air from the steriliser chamber in order to achieve rapid and even penetration of steam into the steriliser load, a steam penetration test shall be carried out each day before the steriliser is used.
“The steam penetration test is carried out using a device having a defined challenge to air removal and steam penetration for the process. For industrial applications, if the saturated steam process uses consistent and defined sterilisation loads known not to inhibit the penetration of steam, alternative methods may be used based on specified physical measurements and a risk assessment of the likelihood of process failure.”6
While the second paragraph may suggest that a daily steam penetration test (air removal test) is not mandatory for some load types, not routinely performing the test involves risk and should be evaluated. Alternative steam penetration verification methods may not be reliable, reproducible, or as readily accepted by regulatory agencies.
ISO/TS 17665-2: 2009. This document provides guidance on the application of ISO 17665-1 and defines the use of daily air removal tests. Section A.5.1 on Bowie and Dick test states: “This test is a steam penetration test, similar to the small load test and intended for daily use.”7
Annex A (Evaluation of a sterilisation process primarily based on the measurement of physical parameters), Table A.3 suggests the daily use of Bowie and Dick tests.
Annex B (Evaluation of a sterilisation process primarily based on biological inactivation and an accompanying mechanical air removal procedure), Table B.1 suggests routine use of air removal tests.
ANSI/AAMI ST79: 2017. Although applicable mainly to healthcare facilities, ANSI/AAMI also recommends the daily use of Bowie-Dick tests for air removal verification. Section 10.7.6.1 states: “The Bowie-Dick test should be carried out each day the steriliser is used, before the first processed load.”8
FDA Guidance for Industry: 2004. In this document, the US agency states: “It is important to remove air from the autoclave chamber as part of a steam sterilisation cycle.” 9
EN 285: 2015. Section 8.1.3, states: “A fail result of any steam penetration test can be caused by an inefficient air removal stage, the presence of an air leak into the steriliser chamber, and/or the presence of non-condensable gases in the steam supply.”10
EU GMP Annex 1, Draft 2017. Section 8.59 states: “When the sterilzation process includes air purging (e.g. porous autoclave loads, lyophilizer chambers) there should be adequate assurance of air removal prior to and during sterilisation. Loads to be sterilised should be designed to support effective air removal and be free draining to prevent the build-up of condensate.”11
Risk mitigation
The frequency of performing the air removal test is determined by evaluating the quality, business, and regulatory risk. The impact of not detecting insufficient air removal during the autoclave prevacuum cycle must be taken into consideration, as the sterility of all materials processed since the last passing test would be called into question.
Inadequate air removal from an autoclave has a direct impact on autoclave performance, and consequently, product quality. Failure to conduct air removal testing on a suitably frequent basis may lead to a lengthy Quality Assurance investigation involving numerous product lots. Depending on investigation results, the worst-case scenario is a non-sterile product and possible product recall.
Equipment remediation requires downtime and lost production, which impacts the overall business. Non-compliance with regulatory expectations and cGMPs may result in citations and other negative repercussions.
In routine production, the probability of not adequately removing air from the autoclave chamber may be low. However, the impact of not detecting inadequate air removal is quite severe, as this has a direct impact on sterility assurance of the materials.
The use of a daily air removal verification test complies with the autoclave manufacturer’s recommendation and is the simplest, least disruptive, and most robust form of detection.
References
- STERIS Technical Tip #5518, “Importance of Air Removal in an Autoclave Steam Sterilization Process”, 2016.
- Bowie, JH, Kelsey, JC and Thompson, GR. “The Bowie and Dick Autoclave Tape Test”. The Lancet, 1963 i:586.
- ANSI/AAMI/ISO 11140-4, “Sterilization of Health Care Products – Chemical Indicators – Part 4: Class 2 Indicators as an Alternative to the Bowie and Dick-type Test for Detection of Steam Penetration”, 2007.
- STERIS Technical Data Sheet, “SteraffirmTM Bowie-Dick Test Pack for Exposure Temperature 121-124oC (250-255oF)”.
- BS EN 867-4, “Non-biological Systems for use in Sterilizers. Specifications for Indicators as an Alternative to the Bowie and Dick Test for the Detection of Steam Penetration”, 2001.
- AANSI/AAMI/ISO 17665, “Sterilization of Health Care Products – Moist Heat – Part 1: Requirements for the Development, Validation, and Routine Control of a Sterilization Process for Medical Devices”, 2006 (R2013).
- ISO/TS 17665-2, “Sterilization of Health Care Products – Moist Heat – Part 2: Guidance on the Application of ISO 17665-1”, 2009.
- ANSI/AAMI ST79, Comprehensive Guide to Steam Sterilization and Sterility Assurance in Health Care Facilities”, 2017.
- FDA Guidance for Industry, “Sterile Drug Products Produced by Aseptic Processing – Current Good Manufacturing Practice”, 2004.
- DS/EN 285, “Sterilization – Steam Sterilizers – Large Sterilizers”, 2015.
- EudraLex, “The Rules Governing Medicinal Products in the European Union”, Volume 4 EU Guidelines to Good Manufacturing Practice Medicinal Products for Human and Veterinary Use, Annex 1 Manufacture of Sterile Medicinal Products, DRAFT December 2017.
N.B. This article is featured in the October 2019 issue of Cleanroom Technology. Subscribe today and get your print copy!
The latest digital edition is available online.

