Production of non-sterile products requires careful bioburden control and risk assessment. Susan Birks reports from the Pharmig Conference in Oxford, on the new rapid tools and the challenges they bring.
Like other sectors, the personal care product manufacturing industry is under increasing pressure to do more with less. Growing economic constraints are putting pressure on raw material and production costs; stricter regulatory and legislative measures for non-sterile manufacturing are being introduced; and meanwhile consumers are looking for natural products that are safe, made in a sustainable way, but with a long shelf life.
These pressures have particular significance for the microbiologist, whose role is to ensure that products are safe and of the right quality. The recent contamination control conference organised by Pharmig for the personal care industry covered many of the new challenges facing those tasked with controlling bioburden, and offered best practice advice from the industry experts. It also addressed some interesting questions in terms of how best to apply the new rapid microbiological methods being adopted by both companies and legislators.
The main role of the microbiologist was encapsulated by keynote speaker and a principal scientist at P&G UK, Dr Kevin Wright: “We have to know the bioburden coming in from raw materials, the bioburden that we are managing [during manufacture], and the bioburden going out in the product.”
Within this process, materials and products are tested to ensure that known pathogens – E.coli, P. aeruginosa, etc – are absent, which is usually a straightforward task. But when an unknown microbe comes up during sampling, it has to be identified; the microbiologist then has to produce a biosafety level for it by finding out what infections it is associated with and the infectious dose. Often, the latter is achieved through a judicious search on Google.
The dilemma facing today’s microbiologists is that the latest microbial detection equipment will flag up anything and everything in a product, said Wright, which then has to be identified and risk assessed, creating a considerable amount of work and data that has to be managed, and which may lead to unnecessary product recalls.
“Our monitoring and release methods for products are still driven by the metrics of quantify and identify for bioburden associated with products,” said Wright. “However, as the release methods become faster and more sensitive, and molecular methods provide more specific identities for isolated microbes, the industry faces a new challenge of how to interpret this data and manage the risks.”
For example, when a German plant found Burkholderia spp. related to Burkholderia cepacia complex (BCC) in a nasal product, the product was quickly recalled.
B. cepacia – although not important for the normal population – is a pathogen that can cause pneumonia in immuno-compromised individuals and so the decision was taken to recall the product.
However, use of the latest identification tools (illumina sequencing and bio-informatics) showed soon after that the contamination was associated with a “forest floor isolate” of Burkholderia, which had none of the pathogenic markers of the infective varieties and therefore would be classified by microbiologists as an “environmental”. It was only through thorough investigation of its pathogenicity that it was realised that the microbe was unlikely to have been of risk to the user. “It looked like an environmental, it thinks like an environmental and it has the genome of an environmental,” said Wright.
While the product recall was the right action in the circumstances, this knowledge would have changed the risk assessment and level of concern for the public.
A second example given was that of E.coli – usually a species that rings alarm bells, yet it has and can be used as a probiotic ingredient. This illustrates that more information about the specific microbe in question is needed to make informed judgments for risk analysis.
“The new knowledge and techniques will change the risk assessment process for raw materials and products in the future,” Wright suggested. It is not just about identifying the species, it is also about understanding the infectivity or pathogenicity of the specific genomed-bacteria in question.
Are we using the best quality metrics, he asked, or can we move from apply, quantify, identify and Google to apply, quantify, identify and “functionalise”?
Inspectorate expectations
Agreeing that “acceptably safe” is a grey area in non-sterile situations, and that there is little good guidance in legislation for non-steriles, Neil Raw, GMP Inspector, MHRA, outlined some of the basic expectations of the regulatory authority when it is moved to take action following an inspection or product failure. He recommended that companies act quickly to take the following steps:
- make a full review of all incidents
- provide details of the implicated product batches
- carry out a risk assessment of the products
- instigate a corrective action plan
- instate routine sanitation
- improve microbiological monitoring
- introduce action limits and reporting
- review processes
Unlike their sterile counterparts, many non-sterile plants are old and adapted facilities, rather than purpose-built, he noted. Many inspection failures result from ageing facilities and equipment and damaged surfaces. “Microbiologists need to be giving management input as to where future investment needs to be made to ensure that facilities are up to spec,” he said.
One of the challenges for non-sterile sites is to know the appropriate level and frequency of monitoring. “We won’t tell you how many sampling points or how to sample. Companies need to work out and document a rationale, as each plant and product is different,” he said. The MHRA wants to see increased use of risk assessment in deciding what levels are safe and what is out of specification (OOS).
Product trends that have increased the challenge for microbiologists were highlighted by Alyson Axe, formerly senior microbiologist at GlaxoSmithKline (GSK) and now on the denture care clinical trials team, such as a cut in preservative levels to achieve “natural” products, the removal of alcohol from products, increased use of natural ingredients, and the use of cheaper raw materials from unproven suppliers.
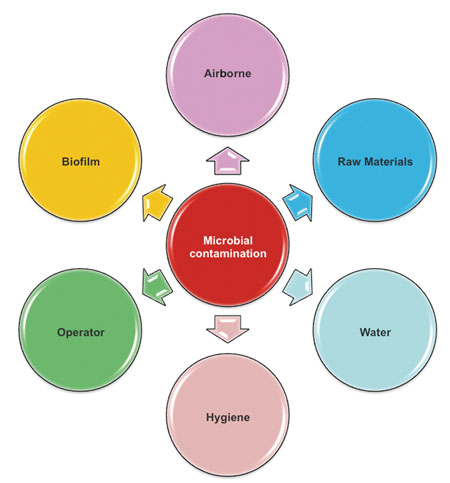
Alyson Axe of GlaxoSmithKline outlined the top six sources of microbial contamination
(Slide reproduced courtesy of GSK)
She outlined common causes of microbial contamination. Inappropriate materials for production equipment, dead legs and dead spots in pipes, storage of equipment in inappropriate areas and poor personal hygiene topped the list, but there were many more. Axe particularly advised the use of stainless steel in place of plastics for pipes and valves. She also emphasised the importance of trending microbiological data, which can ultimately help to “justify a lower incidence of testing”. While testing is important, key to pinpointing contamination hazards was, she suggested, to “think like a microbe”.
Dr Patrick Wouters, Group Quality Excellence – Hygienic Processing, Unilever, looked at hygienic engineering and how production equipment designed for cleanability offers the benefits of being not only safer, but also less costly and quicker to clean. He recommended the European Hygienic Engineering & Design Group (EHEDG) guidelines in this area.
Besides covering the obvious steps of designing out dead legs, crevices and corners, he also emphasised the importance of selecting the right grade of stainless steel, the correct degree of slope for drainage pipes, the need to avoid using ball valves, the correct placing of sampling points, and effective pump selection. He then covered the verification and validation activities that identify contamination issues. He also strongly recommended the use of endoscopes as a tool for inspecting areas that are not readily accessible.
New legislation
As of 11 July 2013, the European Union’s new Regulation (EC) 1223/2009 will require cosmetic products to be manufactured according to Good Manufacturing Practices (GMP). Eize de Boer, international business development manager, Life Sciences, SGS, looked at the practical implications of the ISO 22716 Cosmetic GMP Regulation, which he said covers personnel, facilities, equipment, raw materials, packaging, and treatment of OOS products. He emphasised that risk assessment is at the heart of the document: “Risk management is important and needs to be rolled out to the cosmetics industry,” he said. In the UK, the Trading Standards authorities will enforce the new regulations, and other countries are setting up inspection processes.
Dave Preston, quality manager and Qualified Person, Colgate Palmolive UK, looked at risk management tools such as HACCP and FMEA (Failure Mode and Effect Analysis), while Erika Notman, managing director, Notman Consulting, looked at issues of training for microbiologists.
David Keen, site microbiology manager, Reckitt Benckiser, attempted to take some of the fear out of cleaning validation by explaining the overriding principles. The objective of cleaning validation is “to prove that something is consistently cleaned to be free from product, cleaning residues or microbial contamination”, he said. It should not be performed in isolation but should be part of a company’s QMS; it should also be reviewed on a regular basis.
Having explained the background, his advice distilled down to the following steps:
- Map out the manufacturing process and ensure that all potential failure points are identified in terms of cleaning
- Validate or put in place the test methods first
- Identify the acceptance criteria based on the sensitivity of the test method and the risk to product – chemical, micro and visual
- Risk assess the process to identify what, when and where to sample as part of the validation exercise
- Re-visit cleaning validation if the manufacturing process or product changes.
Joseph Cannata, manager, Global Microbiology Group, Colgate-Palmolive, is a firm believer that real-time data collection gives a much stronger understanding of a facility’s microbial risks. Using clever graphics and an example based on a real incident, his presentation showed the benefit of the real-time collection approach.
He gave two scenarios based on a problem being detected soon after production had started after the Christmas break. In scenario A, contamination shows up in a product sample in the microbiology lab 2–5 days after the initial production. A plant floor investigation is initiated a few days later. The discovery typically sets in motion a flurry of calls and e-mails, while a large amount of inventory is put on hold as the team investigates where the contamination comes from. First to be scrutinised is often the lab itself – was its procedure correct? Then more samples are taken – environmental, surface and in-process material, including more sampling of the production lot. On identification of the contaminants, and now several weeks down the line, there are interviews with the operators and a review of the logs for equipment downtime, and cleaning and sanitisation (C&S) activity to discover what had been done differently.
It is not inconceivable that, after all this effort, the answer will be inconclusive because the data is being collected weeks after the initial production date and the plant conditions are no longer the same, he said.
Alternative procedure
Scenario B, using the alternative of real-time data collection, means that routine in-process material samples are collected from mixers, transfer pumps, storage tanks, transfer manifolds to finishing lines. Such data would have shown that in-process samples were exceeding the set Action level from storage tank 2 and the line A manifold. As a result, the making and finishing supervisors would have been notified and an emergency meeting would be scheduled.
Quality and microbiology departments would have a discussion with operations management. There would be an historic data review that would show that in the previous year action levels were exceeded three times from storage tanks to fillers, enabling the cause to be more readily identified: i.e. the material in storage tanks exceeded maximum hold time, C&S frequency was not followed and an equipment assessment determined that the finishing manifolds were not sanitary.
Additional cleaning steps would then be added to the SOP, and a forward plan to address the Action initiated. Interviews with operators and a review of logs would confirm the product hold time and C&S frequency. Having determined that product X in storage tank 2 exceeded the maximum hold time due to the extended holiday and confirmed that C&S procedure was followed as written, product X could be removed from tank 2. The C&S of tank 2 to line A manifold would be scheduled, the storage tank refilled and production could be quickly restarted, saving weeks of unnecessary panic.
Through the detailed data trending and the generation of micro-organism site maps, the operations team can actively address the problem before it becomes an OOS situation, he suggested. In fact, in scenario B, operation leadership meant that there was already awareness of the problem and area managers were already proactively addressing the issues.




