In nature and in process systems, micro-organisms rarely exist as single cells or even as pure cultures, but rather as a monoculture or a mixed culture of different micro-organisms. Micro-organisms within a biofilm, such as Pseudomonas species, are commonly encased in a slimy matrix referred to as extracellular polymeric substances (EPS), which is essential for the micro-organisms’ survival.
The development and presence of the EPS is very important in increasing the micro-organisms’ resistance to environmental stresses, antimicrobial agents and cleaning agents. Therefore it is critical to remove the EPS prior to sanitisation or disinfection. Surface conditions, such as rouge, can also play a role in removal and disinfection so it is important to consider these when developing a remediation strategy.
It is often difficult to determine the best approach to address the situation, given the myriad of key factors that can influence what products to test and conditions to evaluate. A standardised biofilm reactor methodology combined with traditional cleaning was used to screen numerous factors, such as water temperature, soil condition, commodity chemicals and rouged stainless steel surfaces.
Figure 1 a-c illustrates the importance of distinguishing between microbial residue removal and microbial efficacy. Light microscopy and fluorescently labelled P. aeruginosa were used to show surfaces that were exposed to a biocide and a formulated alkaline cleaner. Viability as measured in fluorescence was lost in both samples, but only the disc exposed to a formulated alkaline cleaner had the visible residue removed.
The authors have carried out research to investigate the cleanability and disinfection difficulties of P. aeruginosa biofilm prepared in a Centers for Disease Control (CDC) biofilm reactor system on both clean passivated biofilm reactor discs and rouged biofilm reactor discs, using total organic carbon surface analysis, visual cleanliness and microbial efficacy testing as acceptance criteria. In addition they have prepared a general biofilm remediation strategy.

Figure 2 a-b: Photograph of (a) CDC biofilm reactor, (b) P. aeruginosa cultured on the CDC Biofilm Reactor disc
Methods and results
A lyophilised culture of P. aeruginosa, ATCC 15442, was used to inoculate 100mL of sterile Tryptic Soy Broth (TSB, 0.3g/L). This culture was incubated in an environmental shaker at 36 ±2°C for 24 ±2 hours. 316L stainless steel coupons were cleaned and placed in the CDC biofilm reactor rods. The CDC biofilm reactor was sealed and 500mL of TSB at a concentration of 300mg/L was prepared and sterilised in the reactor. The reactor was placed onto a stir plate that was set for 125 ±5 rpm.

Figure 3 a-c: Photograph of a) CDC biofilm reactor disc; b) P. aeruginosa cultured on the CDC Biofilm Reactor disc; c) P. aeruginosa CDC Biofilm Reactor Disc after cleaning with a formulated alkaline cleaner
Rouging of CDC biofilm reactor discs: The 316L stainless steel biofilm reactor discs were passivated using a 20% v/v solution of an acidic cleaning agent at 80°C for at least 60 minutes. These passivated biofilm reactor discs were placed in an aerated sodium chloride solution containing rouged mild carbon steel coupons. The biofilm reactor discs were removed from the solution after seven days. The iron level on the discs was determined by using the DR890 handheld HACH colorimeter method 8146. The prepared rouge can be removed with a 5% v/v solution of a formulated acidic detergent at 80°C for 10 minutes with low agitation.
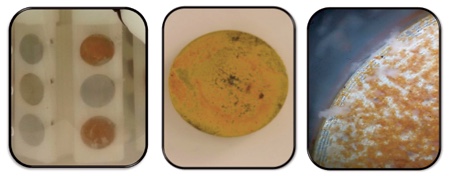
Figure 4a-c Photograph of a) Both rouged and passivated CDC biofilm reactor discs in CDC bioflim reactor. b) P. aeruginosa cultured on rouged CDC biofilm reactor disc, c) P. aeruginosa cultured on rouged CDC biofilm reactor disc under 30x magnification
CDC Biofilm Reactor method: The reactor was inoculated with 1ml of bacteria from the shaker culture prepared previously. The reactor was left in batch phase incubation at room temperature for 24 ±2 hours. 20L of TSB, at a concentration of 100mg/L, was prepared and connected to the CDC biofilm reactor. A continuous flow of the nutrient broth was pumped from the 20L carboy into the reactor using a peristaltic pump set to a flow rate of 11.7 ±0.2mL/min. The reactor was also attached to a 20L waste carboy to ensure a continuous flow of fresh media.
The reactor was operated in continuous flow mode for 24 ±2 hours at room temperature. The stainless steel coupons were harvested by removing the rods from the reactor. Planktonic cells were removed by submerging the rod in sterile deionised (DI) water, ensuring all three coupons were submerged, and rinsed. The coupons were aseptically removed from the rods and transferred into sterile petri plates, making sure not to disturb the biofilm surface.
Microbial efficacy
After incubation, coupons were harvested by first dipping them in sterile DI water then releasing them into a sterile petri dish. Each coupon was transferred to a 50mL conical centrifuge tube. The ASTM single-tube method was used to challenge a formulated alkaline cleaner (1% v/v) at 60°C. Biofilm grown on either clean or rouged coupons was challenged with 4mL of cleaning agents.
To neutralise the reaction, 36mL of cold (~4°C) letheen broth with asolectin and TWEEN (LAT) was added to the 4mL of formulation and vortexed. Each neutralised coupon was subjected to three cycles of 30 seconds vortexing at maximum speed and 30 seconds of sonication, then sampled to obtain serial log dilutions of the neutralised reaction solution. Dilutions were pour-plated with LAT agar and incubated for two days at 37°C.
TOC testing
The P. aeruginosa cultured 316L stainless steel coated coupons were tested wet or allowed to air dry for 24 hours, prior to cleaning. All coupons were evaluated using agitated immersion at 300rpm (Fisher Scientific Isotemp digital hotplate) in a 1,450mL beaker of preheated cleaning solution from ambient to 60°C for five minutes. Formulated alkaline cleaners at various concentrations, biocides, 70% isopropyl alcohol, 6% hydrogen peroxide and DI water were used to evaluate the method’s ability to detect differences in cleaning performance.
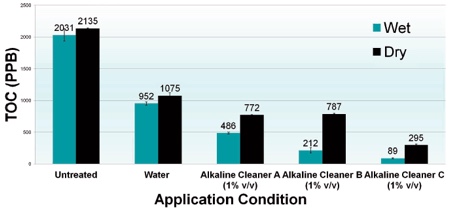
Chart 1: Cleaning wet vs dry residue
Chart 1 compares the residual TOC (ppb) swabbed from the surface of wet and dried EPS discs under three cleaning conditions. The cleaning time of the discs was five minutes at ambient temperature under low agitated immersion conditions
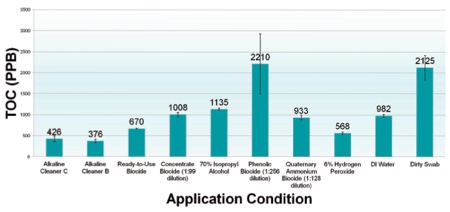
Chart 2: Cleaning vs disinfection
Chart 2 compares the residual TOC (ppb) swabbed from the surface of dried EPS discs of untreated, de-ionised water, select alkaline cleaning agents and select biocides
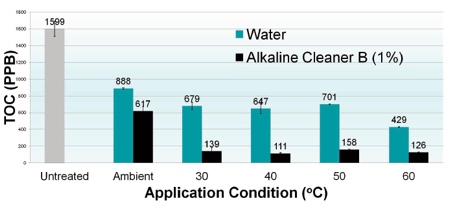
Chart 3: Effect of temperature
Chart 3 compares the residual TOC (ppb) swabbed from the surface of dried EPS discs cleaned with water or an alkaline cleaning agent at various temperatures
After coupons were cleaned for five minutes they were removed from the cleaning solution and rinsed with DI water and swabbed with low TOC polyester swabs (Texwipe). Swabs were placed in TOC vials (Thermo Scientific) and 40g of DI water was added. Vials were then sonicated (VWR Model 250 HT) for 15 minutes prior to analysing for TOC (Seivers 900 Laboratory TOC Analyser).
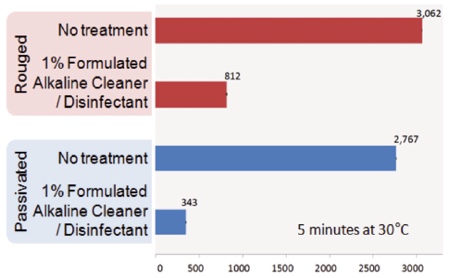
Chart 4: Effects of rouge on cleaning
Chart 4 compares the residual TOC (ppb) swabbed from the surface of dried EPS discs cleaned with water or an alkaline cleaning agent on rouged and passivated surfaces
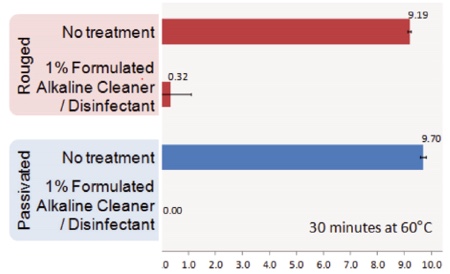
Chart 5: Effects of rouge on disinfectant
Chart 5 compares the log CFU recovered from the surface of dried EPS discs cleaned with water or an alkaline cleaning agent on rouged and passivated surfaces
In conclusion, biofilms present a substantial challenge to maintaining clean processes in validated cleaning environments and it is often difficult to determine the best approach to remove them.
By evaluating numerous factors in association with biofilms grown in the CDC biofilm reactor and using practical experience, we were able to determine general biofilm remediation strategies. These strategies should address both the residue of the biofilm and the disinfection of the equipment. It is necessary to determine cleaning parameters to remove process soil, biofilm, water scale and rouge and then perform disinfection or sterilisation using a product with microbial efficacy claims against the target microbe or microbes.
Bibliography
1. Hall-Stoodley, L. and Stoodley, P. (2002) Developmental Regulation of Microbial Biofilms. Current Opinion in Biotechnology. 13:228–233.
2. ASTM E2562 – 12 Standard Test Method for Quantification of Pseudomonas Aeruginosa Biofilm Grown with High Shear and Continuous Flow using CDC Biofilm Reactor.
3. Buckingham-Meyer, K., Goeres, D.M. and Hamilton, M.A. (2007) Comparative evaluation of biofilm disinfectant efficacy tests. Journal of Microbiological Methods. 70: 236–244.





