At first glance, internal knowledge transfer appears to have many advantages, like being cost-effective. Experts within a company would be theoretically available to train others, and existing procedures have been established. However, due to the complex nature of processes within cleanrooms, and the new requirements of the EU GMP Guide Annex 1, the question becomes: Is internal knowledge transfer sufficient?
The successful implementation of the revised EU GMP Guide, Annex 1 (published in December 2017) requires that pharmaceutical production employees obtain training that is specific to their individual work area. Consider an excerpt from Section 4.3 of the guide, which states:
‘All personnel (including those performing cleaning and maintenance) employed in such areas should receive regular training, qualification (including sampling of the operators bioburden, using methods such as contact plates, at key locations e.g. hands arms and chest) and assessment in disciplines relevant to the correct manufacture of sterile products. This training should include reference to hygiene, cleanroom practices, contamination control, aseptic techniques and potential safety implications to the patient of a loss of product sterility and in the basic elements of microbiology.’

Visualisation helps operators to raise awareness and change their behaviour
Only trained personnel who have passed the gowning assessment and have participated in a successful aseptic process simulation test, during which they performed their normal duties, should be authorised to enter any grade A/B area.' - EU GMP Guide, Annex 1
According to the EU GMP guideline, sound training is about learning the proper methods, which are process-specifically adapted to the users. External training providers are therefore important partners for pharmaceutical companies and make a valuable contribution to the safety and quality of the product.
Pharmaceutical companies with high security levels do not leave the selection of training providers to chance. They hire certified instructors whose teaching methods align with ISO 29990 standards. Currently, there are only a handful of suppliers worldwide with this unique certification. ISO 29990 is a quality management system standard for education providers that makes a critical difference in training, effectively guaranteeing its lasting success. Donald L. Kirkpatrick, an American economist and professor at the University of Wisconsin, aptly stated, "If the trainees do not apply what they learned, the program has been a failure even if learning has taken place."
In-house training performed by an external instructor – like one of comprei’s ISO 29990 certified and internationally active cleanroom experts – can effectively guarantee a successful training course. Critical goals of this training include imparting understanding, awareness, and anchoring behavioural changes. Feedback from numerous well-known pharmaceutical companies has confirmed that comprei’s ISO 29990 certified education program provides an improved learning environment and participant motivation, along with a strong customer orientation. These factors have made comprei a highly desirable partner for leading pharmaceutical companies worldwide.
Acclaimed and Well-Grounded Training

The comprei “cleanroom cuboid” – our mobile training cleanroom
Regulations and internal SOPs provide a solid foundation for employee performance. In sensitive cleanroom environments, these robust guidelines are essential to maintaining the output of high-quality products. The EU GMP Guide, Annex 1 states:
‘The personnel working in a grade A/B cleanroom should be trained for aseptic gowning and aseptic practices. Compliance with aseptic gowning procedures should be assessed and confirmed and this should be periodically reassessed at least annually and should involve both visual and microbiological assessment … only trained personnel who have passed the gowning assessment and have participated in a successful aseptic process simulation (APS) test, during which they performed their normal duties, should be authorized to enter any grade A/B area, in which aseptic operations will be conducted, or are being conducted, whilst unsupervised.’
… training should include reference to hygiene, cleanroom practices, contamination control, aseptic techniques …' - EU GMP Guide, Annex 1

Conveying knowledge also means communicating practical skills
Employee knowledge of detailed operating procedures is essential. In addition, understanding the importance of a procedure – the underlying principles – transforms a well-trained and certified employee into a motivated and mindfully aware member of the company that contributes to its success. This is comprei’s goal – a ISO 29990 certified education provider recognised as an expert in cleanroom technology. Before any training begins, a personalised process analysis is performed to precisely determine customer-specific needs. This provides a detailed template of requirements and forms a solid basis for creating a custom curriculum tailored to customer needs.
Due to proprietary considerations, a hands-on training program in the actual production environment may not always be feasible. In such cases, the relevant processes can be simulated at the comprei cleanroom training facility in Villach, Austria, or in the mobile ‘cleanroom cuboid’, which was specifically designed for this purpose. This mobile training cleanroom brings the practise cleanroom directly to the customer – or to any other desired location. Different scenarios from typical activities can be simulated, their effects visualised, and any needed behavioural changes anchored by the hands-on experience.
Internal trainers vs. external trainers
In order to correctly choose to between using internal trainers or complementing with external trainers, the following must be clarified:
- What results should the training achieve?
- What kind of behaviour would be required of my employees to achieve these results?
- What must they learn to be capable of this behaviour?
- How can the training best reach them, achieving a welcome reaction?
If the trainees do not apply what they learned, the program has been a failure even if learning has taken place. - Donald L. Kirkpatrick
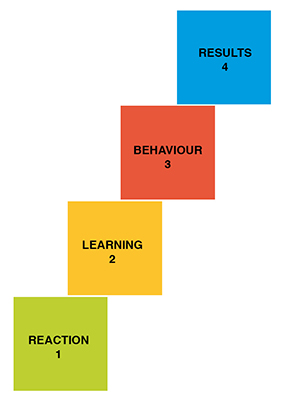
Kirkpatrick model: four levels of training evaluation
comprei’s record of customer and process orientation, practical visualisation and implementation, as well as up-to-date didactics and the certification according to ISO 29990 speaks for itself.
Talk to comprei!
Our training manager Simon Fiala, or an experienced comprei instructor would be pleased to advise you on choosing the right program, in-house training and various modules we offer. Contact Simon on email: simon.fiala@comprei.eu or tel: +43 4242 44075.
comprei programs:
- Cleanroom practices
- Aseptic techniques
- Contamination control
- Hygiene and gowning
