The European Pharmacopoeia (Ph. Eur.) monograph 1927 for highly purified water (HPW) was introduced in 2002 with further discussions and consultation over the years. The revised Ph. Eur. monograph 0169 for WFI was introduced in April 2017. The European Medicines Agency (EMA) issued a question & answer (Q&A) document to provide clarifications and explanations, with the final version published on 1 August 2017. This document did not clear all the questions and even threw up additional ones. At the moment, there are no other applicable, supporting documents available for the EU region.
The EMA Q&A document is a temporary guideline until Annex 1 of the EU GMP currently under revision, is available. It remains to be seen when the revised Annex 1 will be published, and how extensive and detailed the information about WFI with (cold) non-distillation methods will be.
Process design
The correct selection of the process and system dimensions are key to the proper functioning of a membrane-based WFI generation system, and the process combination depends on the quality of the feed water. In addition to the normal parameters, special attention is on the parameters that influence the microbiological quality. These are mainly the Total Organic Carbon (TOC), the temperature and the microbiology (colony-forming units, CFU).
Fluctuations in the quality of the feed water must be observed and taken into account in the process combination. They may be seasonal fluctuations or fluctuations due to having different supply networks with different water quality. The availability of corresponding fundamental data is a key prerequisite for the correct system concept.
Many pharmaceutical water systems are oversized and designed with a lot of reserve capacity
Another important aspect is the capacity. Many pharmaceutical water systems are oversized and designed with a lot of “reserve capacity”. This means, however, that the system is often on stand-by or circulates water internally. This causes a standstill or heating of water, which encourages microbiological growth. The goal should be to design the system so that the production operation is as continual as possible.
The consumption of WFI in production plays a key role here. Determining a consumption profile allows the optimum design and coordination of generation capacity, storage tank size and the capacity of the loop system.
Future capacity expansions can be covered by modular systems and, if necessary, by extra generation units. This provides more safety with regards to microbiological growth, and also more operational safety through redundancy.
The Q&A highlights that selecting the correct pre-treatment procedure makes a key contribution to ensuring that the downstream reverse osmosis (RO) stage works properly. Doing so requires specialist know-how to select the optimum process combination based on the required fundamental data (e.g. feed water analysis).
Reverse osmosis and CO2 removal
While the WFI monograph 0169 states “Reverse Osmosis may be single-pass or double-pass”, in the EMA-Q&A document the double-pass reverse osmosis process is highlighted as an extra barrier and assurance: “Use of double-pass RO membranes should be considered as an added assurance of the maintenance of the quality of the water produced”.
Double-pass osmosis is used in combination with scale control agents. The benefit of this is the extra germ barrier and elimination of the water softener (often microbiologically critical), reducing the microbiological risk.
It is often necessary to remove free CO2 to ensure the reliable operation of the downstream electrodeionisation (EDI). The permitted CO2 concentrations depend on the manufacturer. Membrane degasification is a commonly used process stage for CO2 separation. Air is normally used as a stripping gas for CO2 removal.
Electrodeionisation, nanofiltration and ultrafiltration
The WFI monograph 0169 also states: “Reverse Osmosis, which may be single-pass or double-pass, coupled with other appropriate techniques such as electro-deionisation [EDI], ultrafiltration (UF) or nanofiltration (NF), is suitable.” Normally, the RO/MEG is connected to a downstream EDI, in which the residual desalination and the further reduction of TOCs, SiO2 and CO2 are done.
The EDI modules often used in the pharmaceutical industry are either designed using a plate and frame construction or as a spiral-wound module (see Fig. 1). Table 1 shows the typical values that can be achieved after EDI.
The physical/chemical requirements of WFI are already safely met after the EDI stage. The goal of the last process step is to capture microorganisms and endotoxins/pyrogens.
The NF mentioned in the WFI monograph 0169 and the EMA Q&A was assigned secondary importance in the ISPE workshop. In the last process stage, UF was favoured. The low cut-off of the NF does not offer any benefit, as the retention is ensured by UF (see Fig. 2). Unfavourable operating conditions, e.g. high operating pressure and other issues such as the integrity testability, favour UF, a process that has been used successfully for years in HPW production as a tried-and-tested final preparation stage.
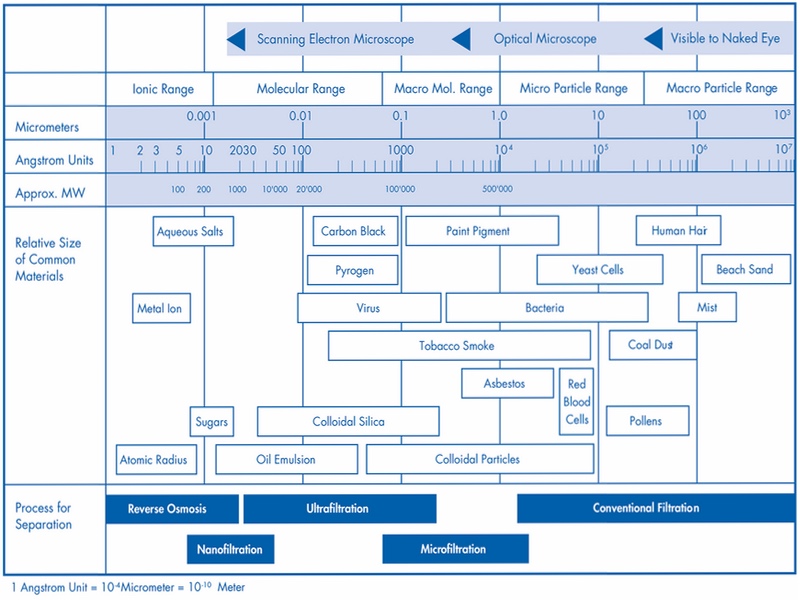
Figure 2: Cut-offs of the different filtration procedures. Source: the author, based on https://bit.ly/2lO3M4k
UF modules come in different configurations such as hollow fibre, spiral wound and ceramic modules, and can be operated in either cross-flow or dead-end filtration modes.
For the separation of endotoxins/pyrogens in pharmaceutical water, ultrafilters are typically used with a cut-off around 10,000 – 20,000 Da. Some UF membranes for pharmaceutical water applications have a cut-off of 6,000 Da. However, it is to be questioned how much better is their retention of endotoxins or pyrogens.
A cut-off safety buffer adjusted to the mode of operation should, however, be provided, as participants in the ISPE workshop reported cut-off changes (increases) to the hollow fibre modules with long service lives. In addition, the cut-off is also influenced by operating parameters such as pressure, temperature and flow-rate.
The actual benefits and risks of UF are therefore always to be viewed in the context of the module type used, the method of operation, the service life and other marginal conditions.
A hollow fibre UF module operated in cross-flow may have a longer service life. With the longer service life, the risk of a cut-off increase, or defect, also increases due to regular pressure fluctuations caused by regularly switching cycles during operation, repeated integrity tests or sanitisation.
With a UF module operated in dead-end mode, this risk is lower due to the shorter service life.
System design
A GMP compliant design is paramount, and a “hygienic” system design is known for reducing the risk of microbiological growth. It also includes the minimisation of dead legs, the optimisation of the pipe routing, drainability, the use of pharma compliant components and connection technology, adequate surface quality for product wetted surfaces (roughness), turbulent flow, welding technology and much more.

Understanding the process and system dimensions are key
to the proper functioning of a membrane-based WFI generation system
A risk assessment shows that even a hygienic design in the individual process stages only achieves limited risk reduction. In water softening, the risk of contamination of the resin bed, due to the large surface of the ion exchange resin, is greater than the risk of an incomplete hygienic design.
In this specific example, other solutions to reduce the risk are key, such as ensuring the operation is as continuous as possible, switching between working and safety columns and regular sanitation.
In water softening, the risk of contamination of the resin bed is greater than the risk of an incomplete hygienic design
With regards to materials, the following is noted in the EMA Q&A: “The materials of construction must not be reactive, additive or absorptive…” In this respect, and also with regards to the surface requirements, no differences can be seen to the general standard of pharmaceutical water systems. The EMA Q&A document mentioned 316l, PVDF and PP, whereby PP is certainly of minor importance in membrane-based WFI generation systems. Gaskets and membrane materials are selected according to the thermal and chemical resistance.
Sanitisation and cleaning
Under the EMA Q&A document, an appropriate routine sanitisation concept is to be integrated as part of the control strategy. A combination of chemical and thermal sanitisation (>75 °C) is recommended, but a corresponding risk assessment and the use of suitable chemical sanitisation procedures are necessary.
The suitable sanitisation cycles to maintain the microbiological quality of the system have to be verified during the qualification of the system. The determination of individual germ species is also noted in the EMA Q&A and should be included in the strategy to adjust the sanitisation concept if necessary.
It also makes sense for individual parts of a system to be able to be sanitised. The main part of the microbiological load is kept back from the RO stage. It should be possible to sanitise them individually, and more regularly, without unnecessarily stressing the downstream stages.
Microbial monitoring
In addition to the normal process parameters, the TOC and microbiology criteria are of key importance for membrane-based WFI generation. TOC represents nutrition for microorganisms, hence the removal of TOC and the corresponding monitoring is crucial.
While the WFI monograph 1069 prescribes “regular monitoring of TOC”, the EMA Q&A requires online TOC monitoring at different points within the system, based on the risk assessment. The quality of the feed water and the selected process combination go into the considerations about which points require online TOC monitoring. If the feed water quality is constant and known, TOC online monitoring of the feed water is definitely not necessary, while it is indicated in the event of seasonal or other fluctuations.
Online monitoring of the TOC after the pre-treatment stage only makes sense if it is designed for TOC breakdown. The TOC retention is largely done at the RO stage. Online TOC monitoring is therefore useful after the RO stage and after the last process stage to verify the WFI quality.
TOC retention is largely done at the RO stage
It should be noted that the current threshold value for TOC of 500 ppb in pharmaceutical water systems is significantly undercut, as TOC values of up to <10 ppb are achieved. To what extent the threshold still makes sense and is up-to-date must be questioned.
The EMA Q&A highlights the importance of rapid microbiological test methods (RMM). The option of online microbiological monitoring offers a lot of benefits because the results are available online in real-time and without delay.
In the event of deviations, appropriate measures can be taken immediately, without losing time on the delays associated with conventional methods. This means, for example, that the continual monitoring of WFI quality and the integrity of the last process stage (UF) are possible.
The respective sanitisation or replacement cycles are to be determined during the qualification. The benefit of the hot water sanitisation is that it allows an automatic procedure while in the event of chemical sanitisation, the rinsing of the sanitisation chemicals has to be checked and qualified.
With appropriate monitoring and monitoring equipment (e.g. differential pressure monitoring of flux for filters, RO or UF stage) it is possible to recognise trends early and introduce preventative measures.
N.B. This article is featured in the October 2019 issue of Cleanroom Technology. Subscribe today and get your print copy!
The latest digital edition is available online.




