While the benefits of Pharma 4.0 are great, pharmaceutical companies may face challenges to digital evolution, such as stringent regulations for budget constraints. In our Horizons: Life Science Report, respondents ranked cost, risk management, security concerns, and skill sets as their top impediments for taking their facility to the next digitalisation level.
However, the Pharma 4.0 operating model considers these challenges and addresses how companies can adopt new technologies into the highly regulated industry while simultaneously considering safety and security needs. We've found that a phased and scalable approach that introduces one digital tool at a time and then adds more as needed generates the best results.
Many pharmaceutical companies strive to add new digital tools into their facility but aren't sure how to do so in a manner that does not disrupt their existing systems. That's when a Pharma 4.0 roadmap comes into play. This roadmap serves as a guide to achieving a company's specific goals - assessing where it is, where it wants to go, and how it can get there.
A roadmap to Pharma 4.0
The first step to creating your Pharma 4.0 roadmap is taking stock of your current situation. Planning a path forward is difficult if you haven't assessed where you currently stand.
One key determination to make is your organisation's level of digitalisation. For example, an organisation that already has a significant amount of digitalisation efforts underway may not need to make the same optimisations or employ the same technology as a company that's still largely paper-based.
Which level sounds the most like your organisation?
Level 1 (Predigital): Uses almost exclusively manual processes and paper-based documentation like SOPs and batch records.
Level 2 (Digital Silos): Uses a mix of manual and automated processes; however, the automated processes do not interact, creating islands of automation.
Level 3 (Connected Plant): Has a higher level of automation and integration and some level of system standardisation.
Level 4 (Predictive Plant): Has an integrated network that allows for real-time predictive analytics.
Level 5 (Adaptive Plant): Is fully autonomous and self-optimising with end-to-end value chain integration from suppliers to the patients. This is a plant of the future.
Essentially, every organisation's situation is unique, and a one-size-fits-all solution won't yield the best results.
Step One: DPMM level assessment
The Digital Plant Maturity Model (DPMM) is a great way to measure your organisation's current level of digitalisation. The DPMM spans from Level 1 to Level 5,x with each level having specific distinctions from one another.
Once you've determined what level your organisation is currently at, you can set a target level you'd like to reach.
Step Two: Evaluate pain points and make crucial considerations
To reach your target level and transition to a Pharma 4.0 operating model, your organisation will need to implement new technology. We've found that introducing new technology works best when applied to specific problem areas.
What are your pain points? What would you like to improve within your organisation? These questions will help you decide which digitalisation use cases are the most appropriate for your organisation and its goals.
There are a number of other questions to consider when creating your roadmap to Pharma 4.0. Some considerations include:
- What technologies are available to your organisation?
- What have you seen other companies doing?
- How will this impact your organisation financially?
- Will new technology affect facility design?
- Who are key vendors and integrators that can acquire and implement this technology?
- How will new technology affect your employees? Will they need further training? Will you need to add to your workforce?
- What are the risks associated with the new technology? Will you need to implement extra cybersecurity measures? Will it affect product safety?
- Can you create a test-and-learn sandbox at an existing site to reduce operational risks and costs for a new facility?
A thoughtful and methodical approach to Pharma 4.0 will ultimately generate better outcomes and help to avoid unwanted consequences.
Step Three: Identifying appropriate Pharma 4.0 use cases
Because there are so many technologies you can add to your Pharma 4.0 roadmap, it's crucial to identify which works best for your specific goals and what you can introduce and use at a sustainable level.
While there are many digitalisation trends companies are using in their journey to Pharma 4.0, here are three examples.
System Integration
System integration is the process of designing your IT infrastructure to allow data to be shared and connected between different systems. There are two types of integration: horizontal and vertical.
Horizontal integration refers to the different systems throughout a facility communicating and networking to collect data and store it in a central location. Because horizontal integration creates a higher level of connectivity, we commonly see it within facilities with a DPMM Level of 3 or higher.
An example of this connectivity is merging operational technology (OT) and information technology (IT). Figure 1 shows the various OT and IT structures that communicate in a connected facility.
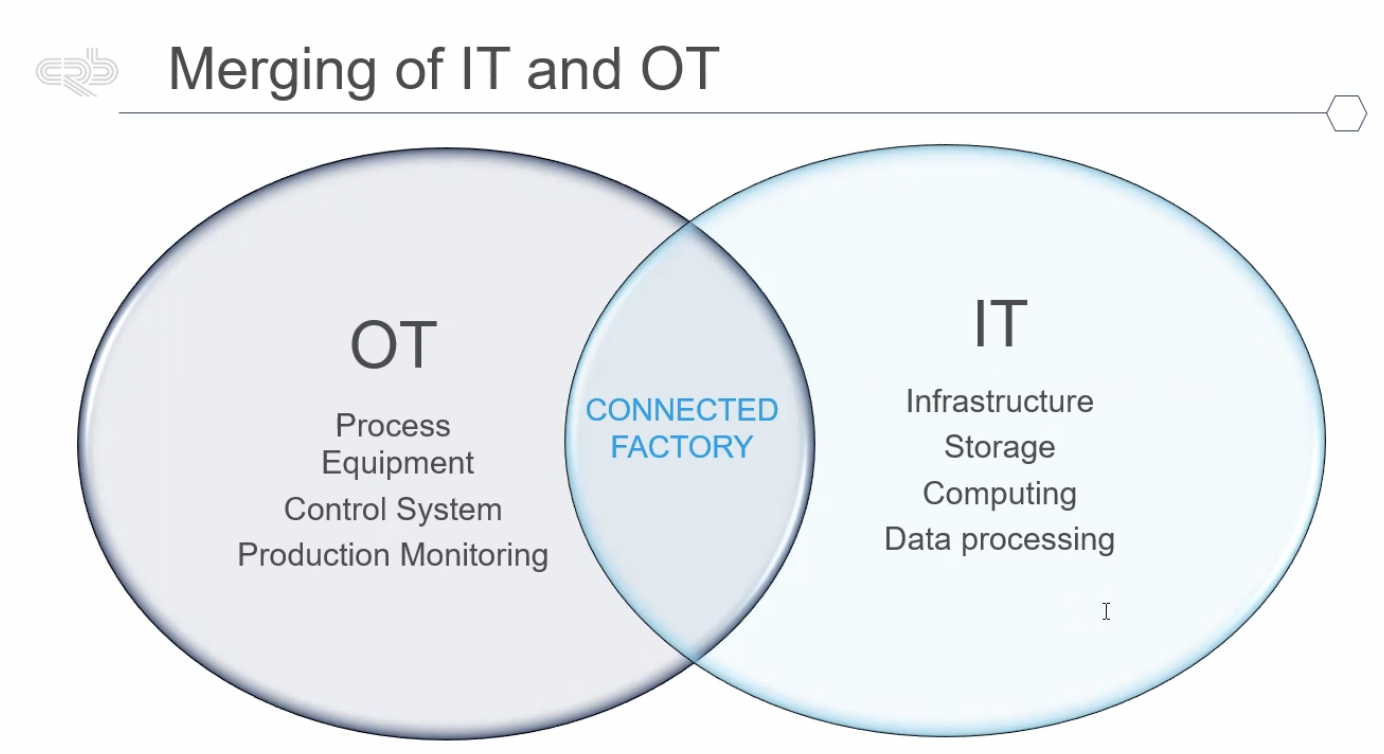
Figure 1: The various operational technology (OT) and information technology (IT) structures that communicate in a connected facility
Vertical integration refers to the ability for a shop floor network and higher-level systems such as manufacturing execution systems (MES), enterprise resource planning (ERP), and laboratory information management systems (LIMS) to communicate and share data. Companies can then use this data to make informed business decisions. Due to the unique communication protocols of many of these systems, setting up vertical integration requires careful planning and execution.
Figure 2 displays a typical automation system and business systems that can integrate to create complete data automation between level 0, which includes physical equipment and facilities, up to the enterprise level.
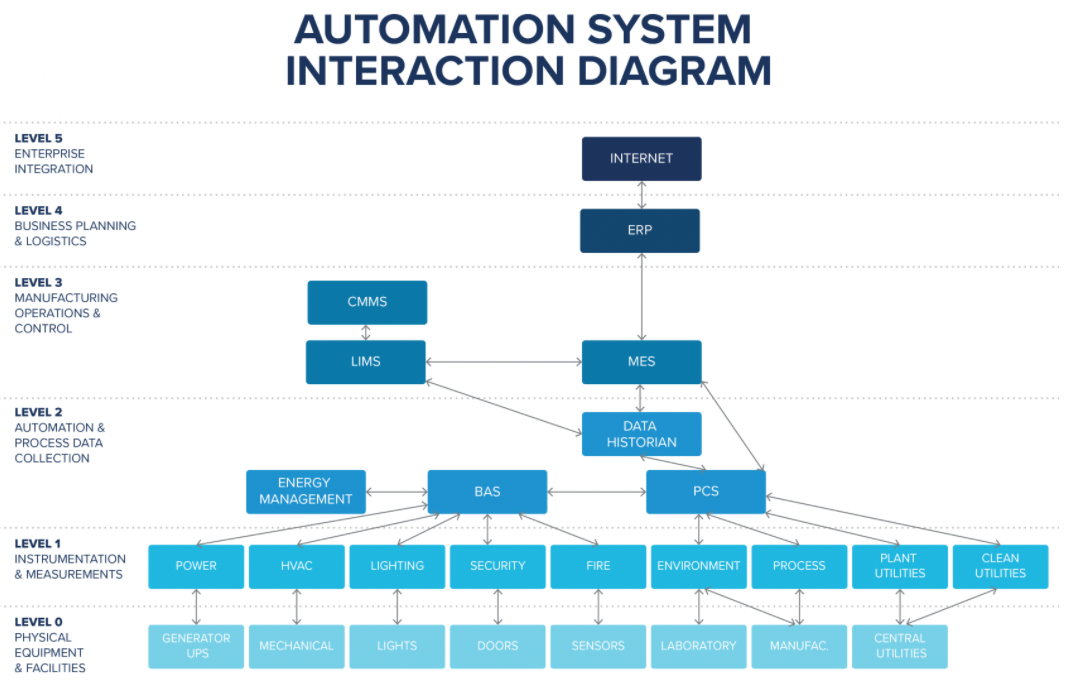
Figure 2: A typical automation system and business systems that can integrate to create complete data automation from level 0 up to the enterprise level
Wearables
The use of wearable technology in pharmaceutical manufacturing has skyrocketed since the beginning of the pandemic. Now that companies have seen the power wearable devices have to optimise various processes, they are here to stay.
"Wearable technology" refers to physical devices an individual wears that allow for virtual reality (AR) and augmented reality (AR) capabilities. Typically, we see this through a device that goes over the eyes and shows a specific scene. In our experience, this scene is typically a 3D rendering of a facility or piece of equipment.
Companies use wearables to streamline training, facilitate remote troubleshooting, and provide electronic work instructions and SOPs. Wearables facilitate effective collaboration with clients, trade partners, and vendors during virtual design and construction (VDC), building information remodelling (BIM), and factory acceptance testing (FAT).
Using wearables, your company and another entity can "walk through" a 3D model design of a facility from different locations, saving time and money without sacrificing accuracy.
AGVs and automated storage and retrieval systems (ASRS)
A great example of making thoughtful choices and considerations before adding new technology to your Pharma 4.0 roadmap is automated guided vehicles (AGV) and automated storage and retrieval systems (ASRS).
Both technologies reduce labour costs, enhance safety, and increase accuracy and productivity but come with a high upfront cost. It may not be economically feasible unless your throughput is over approximately 500 pallets/week.
These technologies may also necessitate design changes, such as ensuring hallways and doorways are wide enough to accommodate them. Companies will also need to determine whether they will have access to elevators.
While the upfront costs and potential design changes may seem like a deterrent, consider the benefits mentioned above that AGVs and ASRS come with: reduced labour requirements and costs, reduced picking errors, increased safety by reducing the number of potential accidents, and better space utilisation.
It's crucial to weigh the pros and cons of each technology to ensure you tailor your Pharma 4.0 roadmap to your organisation's specific needs, goals, throughout, and facility.
Finding Pharma 4.0
While achieving Pharma 4.0 may come with challenges, creating a sustainable, step-by-step roadmap will help your organisation avoid common pain points experienced when introducing new technologies.
The journey to Pharma 4.0 may seem daunting, but the benefits and exciting opportunities created by increased efficiency, accuracy, and safety within your facility are vast.
