STERIS has carried out a lot of research into the removal of biofilms in the past and this latest project shows how the combination of an effective alkaline cleaning detergent to remove the organic residue associated with the biofilm followed by a sporicide can be an efficacious means to address highly resistant biofilm cell populations, such as Bacillus cereus (B. cereus).
The methods for the testing of disinfectants against bacterial single-species biofilms have been evolving recently as the difficulty of biofilm remediation continues to gain much needed attention.
Bacterial single-species biofilm contamination presents a real risk to GMP regulated industries. However, mixed-species biofilms and biofilms containing bacterial spores remain an even greater challenge for cleaning and disinfection.
Among spore-forming microorganisms frequently encountered in pharmaceutical manufacturing areas, the spores of B. cereus are often determined to be the hardest to disinfect and eradicate. One of the reasons for the low degree of susceptibility to disinfection is the ability of these spores to be encapsulated within an exopolysaccharide biofilm matrix.
In a series of experiments, the authors of this paper evaluated the disinfectant susceptibility of B. cereus biofilms relative to disassociated B. cereus spores and biofilm from a non-spore-forming species. In addition, they assessed the impact that pre-cleaning has on increasing that susceptibility.
Method
Preparation of B. cereus ATCC 14579 spore suspension
A broth suspension of B. cereus ATCC 14579 was passed onto nutrient agar supplemented with manganese sulfate monohydrate and incubated for 12–14 days at 36±1°C. After incubation, bacterial spores were separated from vegetative cells and cellular debris by repeated centrifugation, decanting, and re-suspension in de-ionised (DI) water. After processing, the suspension was checked for high spore titre using phase microscopy. The spore suspension was stored at 2–4°C.
Growth of B. Cereus ATCC 14579 biofilm using the CDC biofilm reactor
B. Cereus ATCC 14579 biofilm was prepared following ASTM E2562-12, with modification. B. cereus ATCC 14579 spores suspended in DI water were passed to tryptic soy broth (TSB) (0.3 g/L) in a CDC biofilm reactor assembled following ASTM E2562-12 using polycarbonate coupons (RD 128-PC, BioSurface Technologies Corporation). The culture was stirred at 125 rpm for 24 hours at ambient temperature. After 24 hours, the culture was stirred for an additional 24 hours, at ambient temperature, as fresh media (TSB 0.3 g/L) was introduced at a constant rate of 11.7 mL/min. The reactor maintained a constant volume of media by slowly discarding extra media through wash-out.

CDC Biofilm Reactor disc
ASTM Single Tube Method for assessment of biocide activity against biofilm
The ASTM E2871-13 ‘Standard Test Method for Evaluating Disinfectant Efficacy Against P. Aeruginosa Biofilm Grown in CDC Biofilm Reactor Using Single Tube Method’ (ASTM E2871-13, 2013) was used to assess the effectiveness of treatments in reducing biofilm viability.
Liquid suspension testing for assessment of biocide activity
A liquid suspension study was used to assess the ability of treatments to reduce the viability of spore cell suspensions. Aliquots of a sporicide, containing hydrogen peroxide and peroxyacetic acid, were diluted with DI water to 3% and 12% (v/v). The biocide activity was neutralised by adding an aliquot of the organism/product mixture to chilled lecithin and tween (LAT) broth (with 1% v/v catalase when testing oxidisers) and vortexed. Each neutralised reaction was assayed for viable colony forming units via pour-plating with LAT agar and incubated for 1 or 2 days at 37°C.
Assessment of surface cleaning
Testing occurred at room temperature. B. Cereus ATCC 14579 biofilm on polycarbonate coupons were cleaned by submersion in a stirred, pre-heated volume of an alkaline detergent or a stirred volume of room temperature sporicide (previously described 12% v/v in DI water). The alkaline detergent contains sodium hydroxide, chelants and other components to improve surface wetting, soil emulsification and dispersion of residues. Treated coupons were then rinsed with DI water and either allowed to dry (single treatment), or placed into an additional volume of stirred, room temperature sporicide (previously described 12% v/v in DI water).
Coupons that were cleaned with the second solution (two-step treatment) were then rinsed with DI water and allowed to dry. The dry, treated coupons were then swabbed and those swabs were analysed for adenosine triphosphate content (ATP) (Ultrasnap ATP swabs and SystemSure Plus Lumonometer Hygenia/SS3).
The results
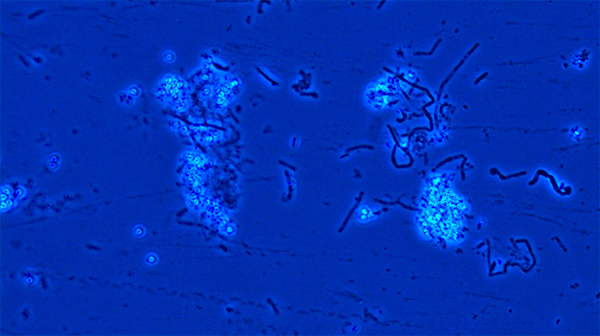
B. cereus spore suspension under 40X magnification using phase contrast microscope
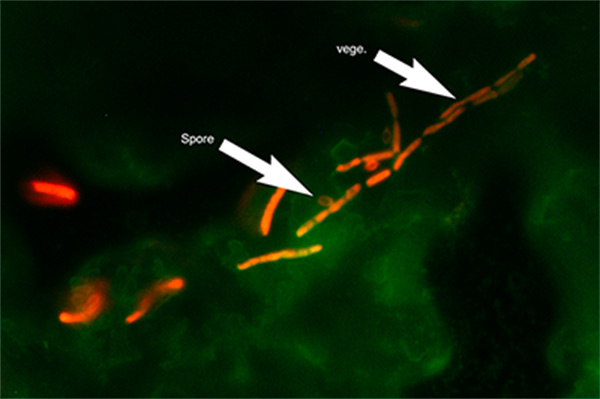
Micrograph of B. cereus biofilm stained with Live/Dead metabolic stain. Composite image of FITC ex/em and Texas Red ex/em illumination. Green coloration: FITC signal. Red coloration: Texas Red signal. ‘Spore’ arrow: bacterial spore morphology. ‘vege.’ Arrow: vegetative cell morphology. 1000x optical magnification
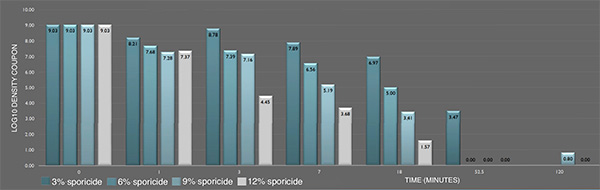
Inactivation of P. aeruginosa ATCC 15442 biofilm prepared following ASTM E2565-12 by the sporicide as assessed by ASTM E281-13. Each data point represents the geometric mean of two determinations. (L-R bars) 3%; 6%; 9%; and 12% sporicide(v/v in DI water)
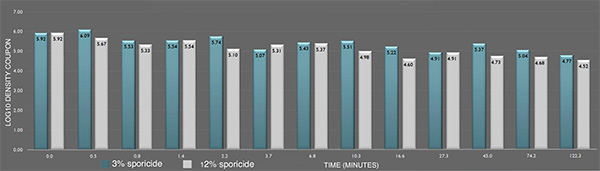
Inactivation of B. cereus ATCC 14579 biofilm by the sporicide. Each data point represents the geometric mean of two determinations. (L-R bars) 3% and 12% sporicide(v/v in DI water)
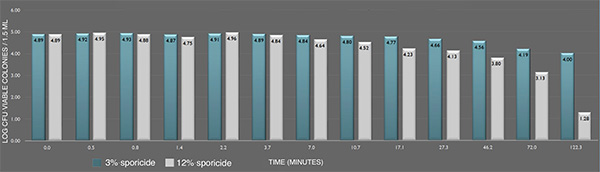
Inactivation of B. cereus ATCC 14579 spores in suspension by the sporicide. Each data point represents the geometric mean of two determinations. (L-R bars) 3% and 12% sporicide(v/v in DI water)
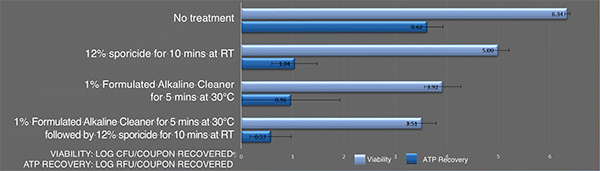
Inactivation of B. cereus ATCC 14759 biofilm by the sporicidewith and without pre-cleaning using an alkaline detergent. Each bar represents the geometric mean of six determinations. Light blue bars: Viable colony forming units recovered after treatment. Blue bars: Recovered ATP represented as relative fluorescence-
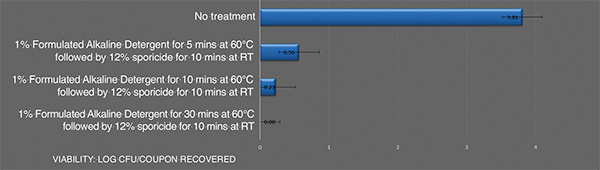
Inactivation of B. cereusATCC 14759 biofilm by the sporicideand using an alkaline detergent. Each bar represents the geometric mean of three determinations. Viable colony forming units recovered after treatment
To access a PDF of the results where the images can be enlarged, please click here.
Conclusions
The conclusions from this study were that the B. cereus biofilm grown under continuous flow and high shear conditions greatly increased the cells’ resistance to inactivation with the sporicide compared to an unassociated spore population. The data highlights the increased resistance associated with biofilm, and even more so the challenges faced when dealing with spore-forming bacteria.
The two-step approach presented here incorporates an effective cleaning step with an alkaline detergent followed by a sporicide to significantly reduce the population of the B. cereus biofilm population.
Combining an effective alkaline cleaning detergent to remove the organic residue associated with the biofilm followed by a sporicide is an effective means to address highly resistant biofilm cell populations, such as B. cereus biofilm. The data shows that increasing the temperature and contact time of the alkaline detergent can lead to a full kill of this resistant biofilm.
*Authors
Amanda Deal, Rebecca Hostettler, Dan Klein, and Paul Lopolito
References
1. Deal, A., Klein, D, Lopolito, P., and Schwarz, J.S., Cleaning and Disinfection of Bacillus cereus Biofilm. PDA J Pharm Sci and Tech 2016, 70 208-217.
2. McDonnell, G. (2007) Antisepsis, Disinfection and Sterilization: Types, Action and Resistance. ASM Press, Washington, DC.
3. Karunakaran, E. and Biggs, C.A., Mechanisms of Bacillus cereus biofilm formation: an investigation of the physicochemical characteristics of cell surfaces and extracellular proteins. Applied Microbial and Cell Physiology. Published online: 9 October 2010.
4. Wijman, J.G.E. et al. (2007), Air-Liquid Interface Biofilms of Bacillus cereus: Formation, Sporulation, and Dispersion. Applied and Environmental Microbiology. 73, (5), 1481-1488.
5. ASTM E2562-12, Standard Test Method for Quantification of P. aeruginosa Biofilm Grown with High Shear and Continuous Flow using CDC Biofilm Reactor, Approved April 1, 2012.
6. ASTM E2871-13, Standard Test Method for Evaluating Disinfectant Efficacy Against P. aeruginosa Biofilm Grown in CDC Biofilm Reactor Using Single Tube Method, Approved 1.10. 2013

