It is well recognised that patient health can be seriously compromised by microbiological contamination of compounded medicines, particularly in the most vulnerable patients. A combination of inadequate facilities, unsanitary conditions and deficient cleanroom practices in countries lacking the necessary regulation, has led to numerous infection outbreaks from contaminated compounded medicines.
Even in well-regulated countries, some companies that fail to monitor and maintain good practice during the compounding process can still slip through the net. For example, steroids for epidural delivery, manufactured at the New England Compounding Centre in 2012, were found to be contaminated with fungal spores leading to over 700 reported cases of meningitis and 64 deaths.1-4
Statutory regs and disinfection measures to kill spores
To prevent such outbreaks, Good Manufacturing Practice (GMP) regulation is applied. In the UK, it has been applied for 49 years, following the introduction of the Medicines Act 1968, then the Human Medicines Regulations 2012.5,6 Compounding Pharmacies have no exemption. The ‘regulatory black hole’7 in United States medicine compounding is now monitored and regulated by the Food and Drug Administration (FDA).8
The trend towards universal standardisation of compounding regulations is welcome, but does not allow for complacency. Although stringent disinfection procedures were followed, Bacillus cereus contamination of intravenous total parenteral nutrition (TPN) resulted in 19 cases of septicaemia and three deaths on a neonatal unit in England in 2014.9 Following an investigation by the Medicines and Healthcare products Regulatory Agency (MHRA), it was concluded that the contamination was introduced into the TPN supplies during manufacture, rather than via any of the raw materials.
The 2014 outbreak in England was described as an isolated incident, however, it highlighted the particular risk of contamination by resilient spores from the environment. Subsequent guidance from the MHRA included the use of sporicides in a two stage spray and wipe disinfection process during compounding.10 This guidance has been incorporated into a report by the NHS Pharmaceutical Micro Protocols Group — ‘Guidance for Aseptic Transfer Processes in the NHS: Addressing Sporicidal Issues’ — which recommends a two minute dwell time with sporicide.11
Assessment of operator aseptic techniques
One of the standard procedures to maintain sterility during compounding is the assessment of operators’ ability to transfer liquids aseptically (see Fig. 1). Bacteriological medium, such as tryptic soy broth (TSB), replaces compounding medicines and the flow through the compounder is examined for microbial growth. Contamination events result in immediate cessation of compounding by the operator concerned, followed by retraining in aseptic liquid transfer. This is essential to maintain standards, providing the validation results are an accurate portrayal of competency.
Following a series of aseptic process simulation validation (compounder media fill simulation) failures from multiple operators at the UK’s Great Ormond Street Hospital (GOSH) pharmacy, it was deemed unrelated to operator competence. All operatives involved displayed excellent cleanroom techniques and practices. However, Bacillus species had been transferred to the Grade A environment in which compounding of parenteral nutrition (PN) is executed, despite strict adherence to the new NHS guidelines that address sporicidal issues.11 An investigation was conducted by GOSH to find the contamination source, evaluate the risk to asepsis of patient doses and identify appropriate corrective and preventative action (CAPA) measures to reduce the risk.
Identifying the cause
The study found GOSH’s media fill transfer procedure to be very rigorous from start to finish. All TSB bottles were received in cardboard cartons and held in an unclassified storeroom until required. Cardboard is known to be a potential reservoir of bacterial and fungal spores and, therefore, kept away from the pharmacy cleanroom facilities. Each bottle removed from the box was sprayed with 70% industrial methylated spirits (IMS) (2 minutes contact time), wiped and placed in the transfer hatch to a Grade C cleanroom.
The bottles were sprayed and wiped again using a stabilised chlorine dioxide and quaternary ammonium compound sporicide. The hold time was increased to 20 minutes from the recommended 2 minutes minimum in NHS guidelines on using sporicides, which is comparable to practice in the pharmaceutical industry. Following transfer through another hatch into a Grade B cleanroom, the bottles were sprayed with 70% IMS and placed in laminar flow cabinets (LFC) (where the BAXA Exacta-Mix EM2400 compounder is situated – Fig. 1) or non-gassed isolators. However, despite this thorough process, media fill simulation samples were found to be contaminated.
A number of different species of Bacillus were identified by Matrix-Assisted Laser Desorption-Time of Flight (MALDI-TOF) mass spectrometry (see Fig. 2) as the cause of media fill contamination. However, uninoculated test bottles of the same batch of TSB used for the validations were uncontaminated and the large bore spike, used to connect the medium to the compounder was supplied sterilised by ethylene oxide and securely packaged. This suggested environmental contamination was transferred into the bottle as the two were connected.
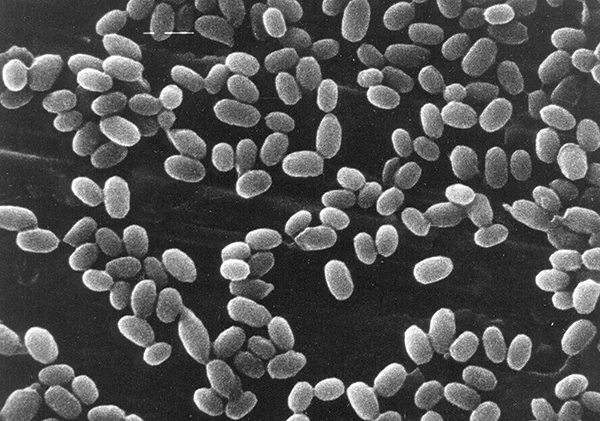
Figure 2: Bacillus subtilis endospores Magnification: x7000
Finding the contamination source
To identify the source of contamination, microbiological samples were obtained from different surfaces within the Grade B environment with localised Grade A protection (LFC), from the Grade A environments and from the surfaces of items subjected to sporicidal transfer disinfection.
The sensitivity of the surface sampling, obtained using 55 mm diameter TSA-filled contact plates, was enhanced by smearing the surface of the agar over the majority of the surfaces being sampled. The TSB bottles were also sampled with contact plates. To ensure all surfaces of the bottle were sampled, the crimped collar was removed so that the part of the bung hidden by the collar could be accessed.
Bacillus species were only isolated from the surfaces of the large volume (500 ml) TSB bottles, transferred in for use in the aseptic process simulations and from nowhere else in the Grade B room (housing three LFC). Speciation confirmed the same Bacillus species as those recovered in the media fill samples. No bacteria were recovered from other large volume bottles, containing licensed drug products, used as starting materials.
How did the contamination occur?
Despite the use of sporicides it is feasible that Bacillus spores on the TSB bottle surface and bung survived disinfection steps during transfer to the compounder. Spray and wipe steps reduce contamination risk during transfer of bottles to the compounder, however, increased handling at each stage is also a risk.
The contents of the 500 ml TSB bottles are aseptically transferred during filling via the insertion of a large bore spike pushed through the rubber septum in the cap of the bottle (Fig. 3). This has a relatively large surface area compared to a needle used for smaller volume transfers. Therefore, any contaminants evading disinfection, such as those under the edge of the collar, may be disturbed and introduced into the bottle at this stage.
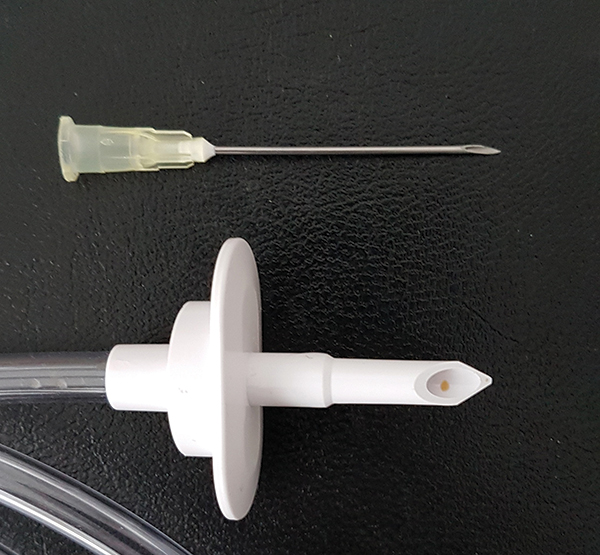
Figure 3: Comparison between large and small bore spikes required to connect large and small volumes to the compounder. There is an increased risk of contamination when connecting
large volumes (500 ml) to compounders because of the large bore spike that has to be
pushed through the rubber septum in the bottle cap.
Could spore contamination occur in actual compounding?
The difference between the recovery of Bacillus isolated on the surface of the TSB bottles and the lack of recovery from the outer surfaces of starting materials was linked to the different manufacturing controls of TSB versus licensed medicinal products. Large volume products used in aseptic compounding are licensed sterile pharmaceutical products, therefore, the bungs are sterilised prior to use, added and crimped in a Grade A environment, reducing the risk of spore contamination (Table 1).
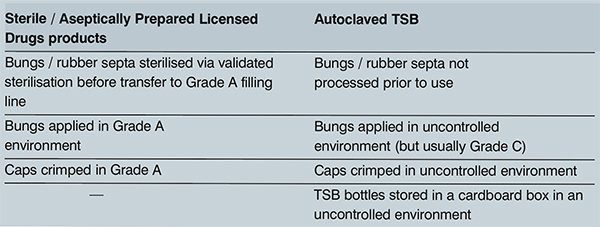
Table 1 Manufacturing controls of licensed drugs for compounding compared to bacteriological medium used for validation
As contamination was only ever found during media fill simulations, rather than during actual drug compounding, this highlighted a potential risk to the sterile environment rather than an immediate risk to patients.
Measures to correct and prevent contamination (CAPA)
Two measures to minimise the risk of contamination of TSB media bottles because of the tabulated differences were identified:
- Increase sporicidal dwell time for both large volume media and licensed products to 20 minutes. This is comparable to the dwell time used for disinfection activities within the pharma industry.
- Identify product that has been triple wrapped and irradiated (discussed below).
The Royal Pharmaceutical Society and NHS Pharmaceutical Quality Assurance Committee have recommended using multiple layers of sterile packaging, reducing handling and the need for disinfection at each transfer stage.12
To provide the same challenge to the aseptic simulation as faced by routine compounding, GOSH decided to determine if sterile multilayer packaging of TSB bottles could eliminate spore contamination of media fill simulation tests. TSB in such a presentation was unavailable, so GOSH approached Cherwell Laboratories (which had already achieved success with triple-wrap packaged and irradiated culture plates) to develop a prototype media bottle with appropriate certification.
Triple-wrapped TSB 500 ml bottles developed
Redipor TSB media (500 ml, in glass DIN bottles with injection stopper and snap cap) was sterilised in a validated autoclave cycle then tested for growth promotion and pH according to the Pharmacopoeia specification. Fig. 4 details the design of these prototype triple-wrapped media bottles.

The first pilot batch was used for a real-time three-month stability trial by Cherwell to confirm that irradiation did not affect media performance after room temperature storage. Irradiation resulted in a slight discolouration of the glass, which was expected and did not affect observations of the bottles post-incubation for medium turbidity resulting from microbial growth. Further batches were made and supplied to GOSH for acceptance testing, including additional samples for the shelf life trial at Cherwell Laboratories.
Preliminary results for prototype use in compounder validation
Assessment is still ongoing, but no ‘false’ media fill contamination events have occurred since the Cherwell prototype has been in use. In addition to it preventing pre-validation contamination, GOSH operators have commented positively about the new packaging, in that it is easy to handle and the polythene bags are easy to remove. One of the main plus points of having multiple sterile layers is removing the need to wipe and spray bottles through transfer hatches. This is saving at least 30 minutes when transferring bottles into the cleanroom. The prototype packaged media has also reduced use of toxic sporicidal sprays, resulting in a safer laboratory environment.
Post media fill and post incubation growth promotion (fertility) testing, using the full range of microorganisms listed in the harmonised methods, was performed by GOSH.13 This confirmed the new medium format still supported growth, even after multiple sterilisation and incubation stages.
Conclusions
Cherwell Laboratories’ triple-wrapped TSB is a faster and more effective way to prevent spore contamination resulting in misleading false results in compounder validation. This prevents unnecessary retraining of fully competent operators, which interrupts workflow and reduces the efficiency of the pharmacy laboratory. It ensures that the media fill simulation procedure is an accurate interpretation of operator competency.
This study highlighted the risk of resilient spores contaminating the compounding process. However, the risk to the sterility assurance of the media fill process was deemed to be greater than the risk to the sterility assurance of routine compounding.
The investigation was able to provide assurance that parenteral nutrition, aseptically filled in the unit, was appropriately controlled. In addition, the aseptic process simulation validation provided a greater challenge to asepsis of the filled product than compounding using licensed starting materials. This meant that there was no risk to patient safety.
Acknowledgements
The authors would like to thank Kathryn Davies (Regional Quality Assurance Specialist, London and South East Specialist Pharmacy Service), Judith Cope (Chief Pharmacist at GOSH) and all the Pharmacy team at GOSH.
References
- https://www.cdc.gov/hai/outbreaks/meningitis-map-large.html. Accessed 26. 4. 2017
- https://www.cdc.gov/hai/outbreaks/laboratory/index.html. Accessed 26. 4. 2017
- http://www.nytimes.com/2012/10/24/health/sterility-found-lacking-at-drug-site-in-meningitis-outbreak.html. Accessed 26. 4. 2017
- https://www.fda.gov/downloads/AboutFDA/ CentersOffices/OfficeofGlobalRegulatoryOperationsandPolicy/ORA/ORAElectronicReadingRoom/UCM325980.pdf. Accessed 26. 4. 2017
- http://www.legislation.gov.uk/ukpga/1968/ 67/pdfs/ukpga_19680067_en.pdf. Accessed 26. 4. 2017
- http://www.legislation.gov.uk/uksi/2012/ 1916/pdfs/uksi_20121916_en.pdf. Accessed 26. 4. 2017
- https://thinkprogress.org/massachusetts-approves-tighter-regulations-to-help-prevent-future-meningitis-outbreaks-e88ff0610e8c. Accessed 26. 4. 2017
- https://www.congress.gov/bill/113th-congress/ house-bill/3204. Accessed 26. 4. 2017
- https://www.gov.uk/government/news/bacillus-cereus-infections-1-july-2014. Accessed 26. 4. 2017
- https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/400232/Guidance_for__specials__manufacturers.pdf. Accessed 26. 4. 2017
- https://www.scribd.com/doc/272972601/Guidance-for-Aseptic-Transfer. Accessed 26. 4. 2017
- https://www.rpharms.com/resources/professional-standards/quality-assurance-of-aseptic-preparation-services. Accessed 26. 4. 2017
- https://www.edqm.eu/en/european-pharmacopoeia-9th-edition. Accessed 26. 4. 2017

