The latest update to the EU GMP Annex 1 regulation (2022) has prompted the need for improved environmental monitoring solutions in sterile medicinal product manufacturing.
While pressure mounts upon manufacturers to increase efficiency and mitigate contamination risks, in-process environmental monitoring solutions like biofluorescent particle counters can help manufacturers to gain real-time insights and boost compliance.
Environmental monitoring (EM) has traditionally been carried out via a growth-based approach, in which air samples are collected, passively or actively, in nutrient media before incubation and analysis.
Although this long-established method is sensitive and effective at detecting airborne microbial contaminants, this testing regime is not continuous and provides only a snapshot of the collected data at a specific time.
Additionally, the time lag between the incubation period and retrieval of results does not allow immediate identification of a contamination event.
Gaining real-time data can support a timely indication of adverse trends
This can have serious implications, as it hinders the potential to implement effective response to reduce the impact of the contamination event on the ongoing process.
The EU GMP Annex 1 regulation was updated in 2022, and came into operation in August 2023.
This represents the first revision of the guidelines since 2008, and as such, the new regulation stipulates that manufacturers of sterile medicinal products meet a host of additional stringent requirements, in-line with the most up-to-date best practices.
For Grade-A classified cleanroom environments, the updated regulation stipulates for a continuous EM regime, and a zero-tolerance limit for colony-forming units (CFUs).
To help manufacturers navigate the transition to the revised Annex 1 regulation, Cherwell recently invited EM specialist, Allison Scott, Ph.D., to share her insights and expertise as part of its “Delivering Knowledge” presentation series.
BFPC technology stands as a ready solution that can comply with these new regulatory requirements
The presentation brought attention to real-time, biofluorescent particle counting (BFPC) technology as a solution for continuous EM, and how BFPC platforms, such as the MicronView BioAerosol Monitoring System (BAMS), can support Annex 1 compliance.
In this article, we will cover some of the key highlights from the presentation, and outline the benefits of rapid microbial monitoring (RMM) technology for improving cleanroom surveillance and detecting contamination events.
Biofluorescent particle counters explained
BFPCs are specialised instruments designed to detect and quantify airborne non-biologic and biological particles, such as bacteria and fungi, via a non-growth-based approach.
In order to detect airborne biological particles using BFPC, the instrument relies on the unique fluorescence properties of compounds within microorganisms.
Most biological particulate contaminants naturally produce fluorescence compounds, such as riboflavins or nicotinamide adenine dinucleotides (NADH), which emit detectable fluorescence when excited by a laser at 405 nm wavelength.
BFPCs are designed to take advantage of this natural fluorescence to detect microorganisms present in air samples (Figure 1).
Although BFPC has been available for use for EM applications in the pharmaceutical industry for more than a decade, the technology is now growing in popularity as a rapid microbial monitoring (RMM) solution for sterile manufacturers for its continuous monitoring capabilities.
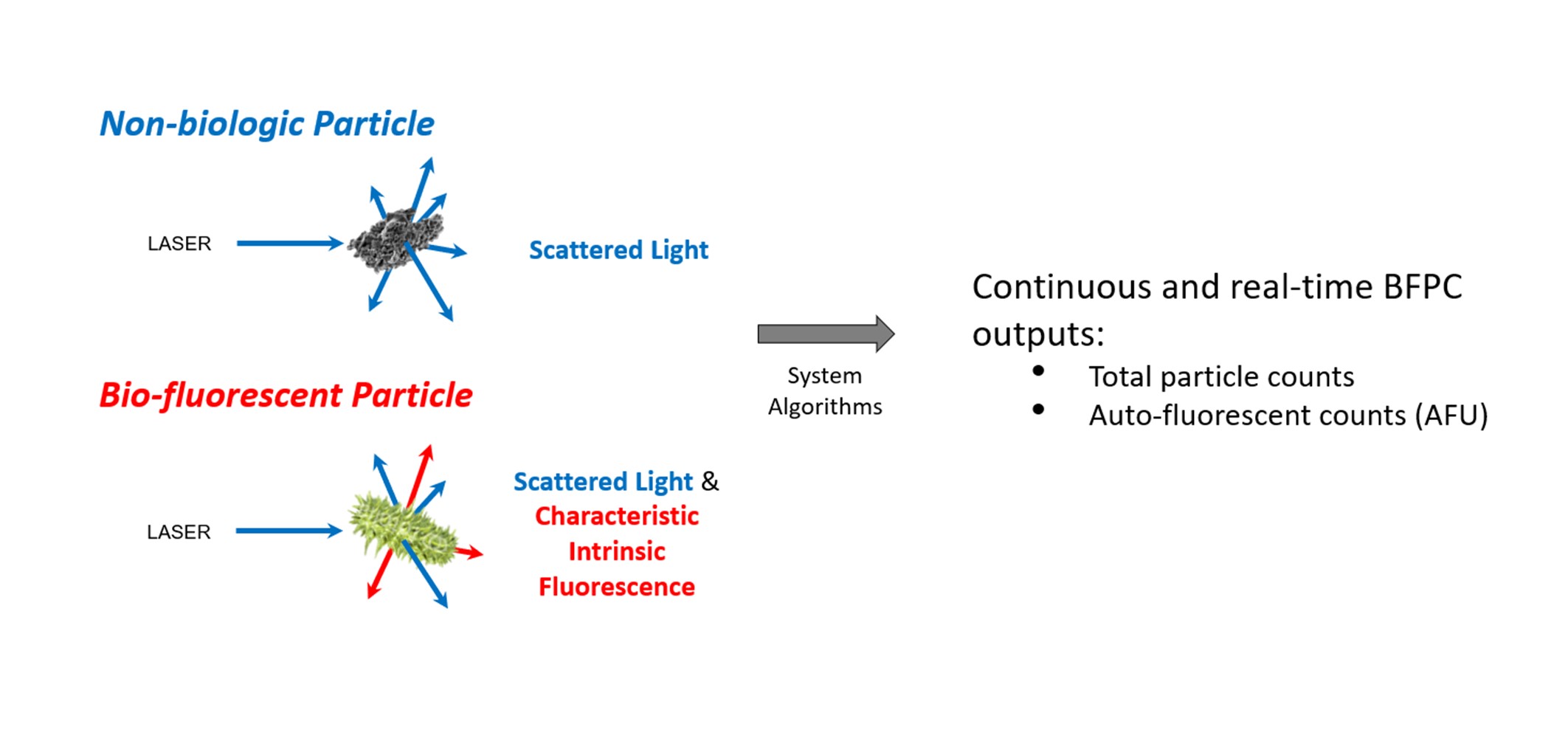
During operation, air is continuously drawn into the BFPC system for analysis. Particles in the sampled air are then exposed to a laser at 405nm and simultaneously assessed for scattered light and fluorescence: (i) scattered light, used to size and enumerate particles; and (ii) fluorescence, for biologic characterisation of the particles.
For all particles within the BFPC system’s detection range, the input light will be scattered and the BAMS will size and enumerate the particles.
Particles that fluoresce under 405nm light, such as bacteria, will undergo laser-induced fluorescence. This emitted fluorescence typically has a longer wavelength than the excitation light and is specific to the particle's characteristic intrinsic fluorescence [4].
Although some non-biologic materials also fluoresce when excited by 405nm light (e.g., some plastics, paper, and glass), BFPC algorithms are designed to minimise characterisation of such interferent materials as biologic.
The BFPC's software analyses the scatter and fluorescence signals to detect and count the airborne particles.
AFU is often a more sensitive measure than the traditional CFUs
The data outputs are given as total particle counts, and Auto Fluorescent Units (AFU) – a unit based upon both size and fluorescence of particles detected.
AFU is often a more sensitive measure than the traditional CFUs (colony forming units), as AFU also accounts for viable but non-culturable (VBNC) microorganisms that would not be detected with agar growth methods.
Advantages and limitations of BFPC
BFPC offers several advantages over traditional agar-based EM methods, and can be of great value to manufacturers seeking to improve their EM regime.
One of the major benefits of BFPC is the ability to perform continuous, in-process monitoring, of both total particles and AFUs.
This can support data trending over time: While agar methods analyse an air sample at a given timepoint, BFPC provides a comprehensive and continuous picture of air quality.
This can help manufacturers better understand their process, and to identify any operational weaknesses that could lead to a contamination event.
Another key benefit of BFPC is the continuous access to real-time data readouts which facilitates immediate detection of airborne contaminants, such as bacteria, fungi, and other particles of interest.
Not all cleanroom spaces require continuous EM
Unlike agar-based methods that require sample collection, incubation, and manual colony counting, BFPCs automate the process, providing rapid microbial monitoring (RMM) and reducing the risk of missing or underestimating contamination events.
Gaining real-time data can support a timely indication of adverse trends to help manufacturers identify root cause faster and subsequently implement corrective action to maintain air quality and product integrity in cleanroom environments.
BFPCs also bring operational simplicity to the table – the vast majority of operations are automated, meaning that little training is required, as well as minimising staff presence in critical cleanroom areas. So, operational risk of contamination is reduced.
Although BFPC can bring a myriad of benefits to EM, there is still an important role for the traditional agar-based approach to play. BFPCs do not have the ability to identify microorganism species – as such, it would be beneficial to gain deeper insight into any contamination detected via BFPC with a follow-up agar plate investigation.
Furthermore, not all cleanroom spaces require continuous EM, and for some lower-classified environments, agar growth remains a completely satisfactory and low-cost EM measure.
A continuous solution for Annex 1 compliance
The latest revision to Annex 1 now demands much higher EM standards in Grade A cleanrooms, including the need for a continuous air monitoring regime to prove stringent cleanroom surveillance and reduced contamination risk.
For organisations looking to introduce a continuous EM system into their Grade-A environments, BFPC presents a viable GMP option. To better understand this, let’s take a look at exactly what the latest Annex 1 documentation stipulates:
Section 9.17 states: “The Grade-A area should be monitored continuously (for particles ≥0.5 and ≥5 µm) and with a suitable sample flow rate (at least 28 litres (1ft3) per minute)”
Section 9.24 states: “Continuous viable air monitoring in Grade-A (e.g., air sampling or settle plates) should be undertaken for the full duration of critical processing, including equipment (aseptic set-up) assembly and critical processing. A similar approach should be considered for Grade-B cleanrooms”
Section 9.28 states: “The adoption of suitable alternative monitoring systems such as rapid methods should be considered by manufacturers in order to expedite the detection of microbiological contamination issues and to reduce the risk to product”
FPC technology stands as a ready solution that can comply with these new regulatory requirements
There is a heavy focus upon continuous total particle and viable monitoring for Grade-A environments, and encouragement for continuous monitoring of other environments too, which is a significant departure from the previous revision.
Furthermore, data trending is stressed throughout the document to support Quality Risk Management, timely indication of adverse trends or a change in the environment, and potential for faster resolution if identified.
Finally, the new document encourages the adoption of alternative monitoring methods, especially RMMs that can quickly identify contamination events.
BFPC technology stands as a ready solution that can comply with these new regulatory requirements: It can be used for continuous EM, provides a greater level of sensitivity than agar-based methods, gives real-time data for rapid detection of contamination events, and gives readouts in a comparable (and indeed more sensitive) unit of measure to the standard CFU. Following an industry summit in 2021, established industry working groups around the globe have been collaborating on the implementation, testing and validation of BFPC systems to support broader adoption and awareness. Further applications of BFPC in development include water analysis, rapid microbiology applications, and additional air monitoring applications for process monitoring and fill-finish manufacturing.
Enabling continuous monitoring
Having operated in the cleanroom technology space for over 50 years, Cherwell maintains a keen interest in evolving industry requirements.
Recognising the need to offer a full suite of specialist EM options depending on individual needs, Cherwell recently introduced the MicronView Bio-Aerosol Monitoring System (BAMS) continuous air monitoring platform (Figure 2).
This BFPC is an ISO-certified particle detector that detects inert and biological particles with high sensitivity to move towards developing compliance to the requirements specified in GMP Annex 1.

Figure 2: The MicronView BioAerosol Monitoring System (BAMS)
BAMS represents the first portable technology of its kind, condensing the latest BFPC hardware into a lightweight (5.8 Kg) benchtop instrument.
Operated via a touchscreen compatible with latex gloves, BAMS can be deployed in various critical locations to deliver continuous monitoring, trend analysis and real-time results.
BAMS should still be used in conjunction with agar-based air sampling methods
As suggested in the latest Annex 1 guidance, in order to achieve a comprehensive EM setup, BFPC technology, such as BAMS, should still be used in conjunction with agar-based air sampling methods.
This will empower users with real-time indication of airborne contaminants, along with the ability to culture and identify the fungus/bacteria species responsible. From there, the best course of action can be implemented, with minimal disruption to production and a reduced risk of product loss.
A new era in sterile manufacturing
Following more than a decade of successful implementation in the pharmaceutical industry, BFPC is gaining popularity in sterile manufacturing EM, providing a host of benefits whilst offering a complementary tool to traditional culture-based methods.
From being a continuous solution, to providing real-time results – the technique can help aseptic manufacturers to gain instant identification of contamination events, delivering alerts in-time to allow rapid remediation to reduce the risk of product loss and ensure the delivery of safe products.
BFPC solutions, like the Micronview BAMS, can help to boost cleanroom standards and meet the new EU GMP Annex 1 requirements.
However, this has not reduced the value of traditional air sampling methods, since cost effective identification of contaminant species remains an important part of understanding and combatting contamination events.

