Microbial air sampling has always been a key part of any environmental monitoring (EM) programme, however, changes required by the latest revision of EU GMP Annex 1 have now upped the ante with regard to ensuring its accuracy. Organisations are now required to monitor cleanroom environments even more precisely to be able to demonstrate that the monitoring meets these new more stringent standards for the manufacture of sterile medicinal products.
Having been undertaking servicing and calibration of air sampling devices for over 35 years, Cherwell’s calibration engineering team had seen evidence that choice of plated prepared media can affect air sampler calibration results in some circumstances. This can in turn render any reading from a device inaccurate and non-compliant, therefore, Cherwell microbiologists undertook a study to investigate further to understand how inaccuracies might arise and share results for wider discussion.
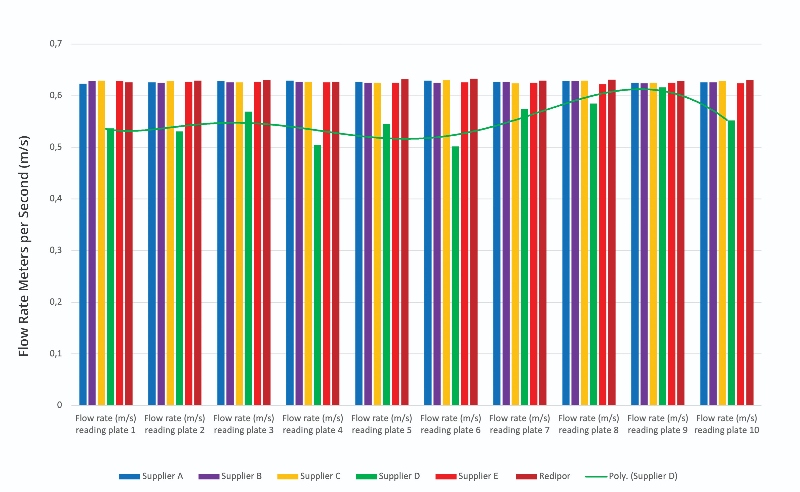
Figure 2
The study compared prepared media plates from the leading manufacturers, testing them against each other via their performance in an SAS air sampler, a device which is routinely used in many facilities. As manufacturers of the Redipor (registered trademark) range of plated media, Cherwell do declare an interest. However, for this exercise, the investigating team were completely impartial in their work and findings, aiming through these to encourage others to conduct their own studies in order to promote further discussion, investigation and understanding on this important topic.
This article presents details and results to date of Cherwell’s plate study investigation asking the question, “Does the choice of plate affect the air sampling results achieved?”
Method and equipment used
The study tested the relative performance of plates from six leading prepared media suppliers using a standard set-up of an SAS air sampler. For the investigation, an SAS Super 180 isolator air sampling head was calibrated according to the Cherwell in-house method. The calibration method required the use of a standard resin contact plate and a standard petri dish to perform the calibration. Resin-filled control mock plates were also used as constants within the process.
The air sampler was powered by a power supply with maintained voltage to produce a constant flow rate of 0.62 m/s, thus ensuring that it ran at 180 L/minute and sampled 1000 L (1 m³) in accordance with Annex 1 requirements. A calibrated anemometer (Schmidt probe) was also used to precisely measure and verify the air flow rate.
After calibration, the following tests were undertaken:
- For each supplier in turn, a contact plate was placed in the unit (shown as the lead picture) and the air sampler was run for five minutes and thirty-three seconds to achieve a sample volume of 1000 L, based on a flow rate of 180 L/minute
- This exercise was repeated ten times for each type of plate, to ensure reliable data
- All flow rate data was captured in a spreadsheet
- These tests were then repeated with the petri dishes (90 mm) from each media supplier
Results
The results from the flow rate reading on the contact plates are shown in Figure 2. These results highlight the variation in maintaining a 0.62 m/s air flow with different plate suppliers. The largest fluctuation in results can be observed in Supplier D’s results which clearly demonstrate an impact on the flow rate of air. In addition, the associated trendline is polynomial, showing the fluctuation in data, and hence inconsistency in the results from Supplier D's plates.
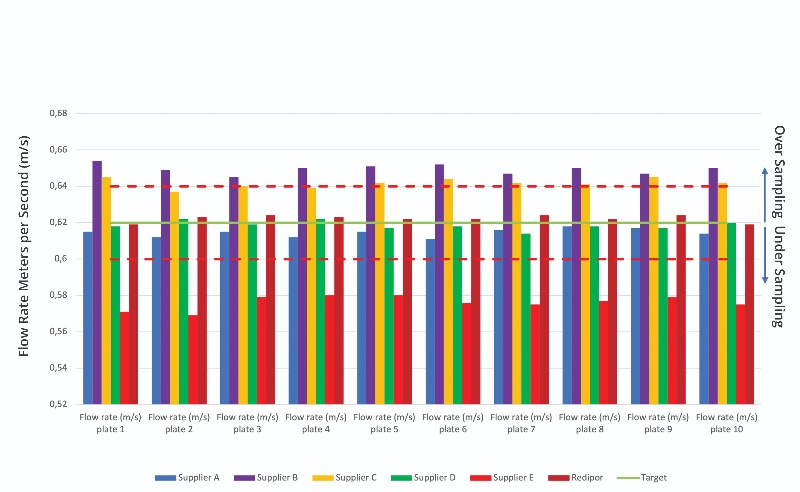
Figure 3
The results of the petri dishes (90 mm) also show varying results against the required flow rate set by the Redipor control (Figure 3):
- Suppliers A and D presented a slight reduction in flow rate, with inconsistencies between samples
- Suppliers B and C demonstrated greater flow rate, with inconsistencies with each sample
- Supplier E showed significant decrease in flow rate
As there are minor fluctuations in the control plate readings, the tolerance allowed for calibration in accordance with procedure is ±3%. The data from both the contact plates and petri dishes were averaged to produce a mean result on flow rate and convert metres/second to litres. The calculation used, as well as final calculated volume consumptions for both contact plates and petri dishes, are detailed in the study report.
Discussion of findings
The results show that air flow rate can definitely be affected by the type of plate chosen.
Contact plate observations
In particular, it was noted that results for the contact plates of supplier D show an air sample volume loss of just over 100 litres (10%) which can cause under-sampling. This can be linked to the height of the plate as the proximity of the sampling head can cause restrictions in air flow, as shown in Table 1 and Figure 4. This extra height is because additional plastic moulding around the agar, which is part of the lid locking mechanism, elevates it on the housing platform.

The impact due to change in flow rate can also be physically seen in the impressions left on the surface of Supplier D’s plates (Figure 5). The lower flow rate delivers a reduced sample volume which is evident in the left-hand image. This is because air in the middle of the plate is not impacting the agar at a high enough speed to make the indents in the centre of the plate due to restricted flow.
In addition to the flow rate which can affect effective sampling, misalignment of media due to the diameter of a plate can also cause potential sampling issues. For example, Supplier A had a larger base (69mm compared to all other plates sampled which are 65-66mm – see Table 1) and, therefore, did not align to the sampler as shown in Figure 6. This required adjustment to enable effective sampling and ensure that all particles impact the agar.
Petri dish observations
The petri dish results also show varying degrees of sample volume. Two brands are marginally and one brand is notably under-sampling due to reduced flow rates, and two are significantly over-sampling. This variation in petri dish flow rate could be due to a difference in fill volume. The standard fill in the Redipor control dish is 18 ml, and any comparative variation on this in other plates would change the height of the agar and consequently affect the air flow rate.
Over and under-sampling risks
Under-sampling carries the risk of failing to detect bioburden with serious associated consumer health implications. Whereas, over-sampling will have an impact when trying to determine the levels of background flora in lower graded areas of the facility. This is because over-sampling increases the possibility of a false positive result, which may result in poor microbial action and alert limits. These in turn will lead to unreliable data, thus delivering false information on a facility’s environment.
Ultimately, an unreliable understanding of the environment of a facility has subsequent critical implications for capture of a possible excursion, microbial contamination of product, and consequent repercussions.
Annex 1 implications
Due to these risks associated with over- or under-sampling, unless properly undertaken, a sampling process may fail to comply with the latest revision of EU GMP Annex 1, released on 25th August 2022, which requires better risk assessment and risk management.
Annex 1 now provides clarity on the required CFU values for microbial contamination during qualification of cleanrooms. It also clarifies action limits from viable particle contamination in all graded areas. However, to achieve the required standard, it is essential that the specified air volume of 1000L (1 m3) is sampled precisely, which requires the correct air flow.
As a result of the serious associated risk that viable organisms within the environment will not be captured, Annex 1 demands that the sampling taking place should demonstrate consistency and accuracy, which in turn provides confidence that a clean room is under control.
Conclusion
The testing confirmed that air sampling results can vary across different plate types, due to variations in size and form factor of the plates which can disrupt air flow through a sampler. This consequently causes over- or under-sampling in some cases, thus affecting results and data to potentially lead to inaccurate readings and lower the consistency of EM reporting.
From this, the study concludes that plate choice for microbial air sampling in EM programmes does make a difference. Therefore, for compliance with the latest revision of Annex 1, sterile medicinal product manufacturing organisations will need to ensure that their chosen plate, in combination with their chosen air sampler, is definitely providing an accurate result.
The use of contact plates or petri dishes which deviate from the recommended standard to which an air sampler is calibrated will have an impact on the flow rate of air, with subsequent risk of producing unreliable data. Therefore, it is important that manufacturers undertake their own investigations to ensure equipment compatibility.
Finally, further recommendations to reduce risk in air sampling include that sampler calibration against established standards should be at least annual to minimise incorrect sampling. Crucially, this calibration should include testing in conjunction with the prepared media plates routinely used by an organisation.

