Aseptic production, especially continuous production with high throughput, requires that the clean area be supplied with materials and that materials also be removed from the clean area. These transfers are among the riskiest steps in the entire pharmaceutical production process, as they pose the risk of introducing suspected contamination into the critical cleanroom class (A-zone, resp. ISO Class 5) where the open product is processed.
The options for transferring products and materials into or out of classified areas vary greatly and depend, among other things, on the type of material being transferred (e. g. product, packaging material) and the cleanroom/barrier system. However, what they all have in common is that maintaining the aseptic barrier is always the top priority when transferring products between different classified areas.
What is aseptic transfer and its regulatory requirements?
Over the past two decades, isolator technology has deservedly positioned itself at the forefront of the safest production environments in the pharmaceutical industry [1], both in the manufacturing and quality control of sterile medicinal products. This is also reflected in Annex 1 of the EC GMP Guide published in August 2022, which explicitly recommends the use of barrier technology, as it significantly increases product protection against potential contamination in the form of endotoxins/pyrogens, particulate matter, and microbial contaminants caused, for example, by personnel, materials, or the environment [2].
Consequently, Annex 1 of the EC GMP Guide requires explicit justification if other environmental conditions are to be used, which gives an additional boost to barrier technology. In a direct comparison of the different barrier systems, isolators represent a closed and strict physical barrier between the environment and the aseptic area where the sterile product is processed. During production, access to the interior of the isolator is only possible via gloves (in case it`s not a gloveless isolator) and validated transfer systems.
The transfer of materials must be considered a critical process step, as it always carries the risk of introducing contamination into the clean area [3]. It is immediately apparent here that maintaining the aseptic barrier when transferring material between different classified areas is of utmost importance in order to ensure the aseptic integrity of the entire process. This applies equally in both directions, both for the transfer process, in which materials are introduced into the isolator, and for the processes in which materials are removed from the isolator [4].
Key regulatory requirements for transfer systems can be derived in particular from Annex 1 to the EC GMP guidelines (2022) [2], where material transfer is considered very important. The requirements described are all aimed at preventing contamination. This is the common goal, regardless of which transfer methods are used.
Since humans generally represent the greatest potential contamination risk in all clean environments and in all aseptic procedures [5], humans should be kept out of the process wherever possible.
Automated processes minimise human interventions and also achieve a level of standardisation that is completely impossible with manual execution. To enable a safe aseptic transfer into or out of the barrier system, various technical options are available which either include an effective decontamination method during the transfer process or a safe introduction of pre-sterilised components. The selection of the most suitable method depends on the material to be transferred or the technically preferred transfer properties.
The currently used transfer systems, as technical solutions for introducing packaging and other materials or tools required for the pharmaceutical production process, can generally be differentiated based on three technical discriminatory factors:
Continuous vs. batch wise
If it is important to provide large quantities of material for immediate and continuous use in the production process (continuous process at a sufficiently high speed), then the choice is to use a continuous transfer system. One example of this is the continuous introduction of the primary packaging into which the product is to be filled. If material is only required as needed, a transfer method that enables batch wise transfer is appropriate.
In vs. out
In addition to the introduction into the isolator, the removal from the isolator also represents a transfer process that must be just as safe, reliable, and efficient. Both the introduction and removal can be carried out continuously or in batches, depending on the transfer system used. Some systems are suitable for transport in both directions, while others are designed for transport in only one direction (i.e., introduction or removal).
With vs. without decontamination
Regardless of whether the transfer is continuous or batch wise, a decontamination process, e.g. with H2O2, can also be carried out simultaneously. Other solutions use transport containers that have been previously sterilised with moist heat, while others employ pre-sterilised single-use systems. An example of a transfer that includes a decontamination step with H2O2 decontamination is the batch wise introduction of material via a Material Transfer Isolator.
Thus, from a technical perspective, 8 groups can be formed using the 3 discrimination factors mentioned above. Instead of classifying the individual material transfer methods based on their technical properties, it may be more practical to classify them based on the materials being transferred:
- Packaging material
- Liquids
- Finished products
- Materials (tools, devices, …)
Figure 1 provides an overview of the most common solutions for aseptic transfer. With the help of the “Aseptic Transfer Advisor (ATA)” shown here, you can see at a glance which transfer system is suitable for which application.
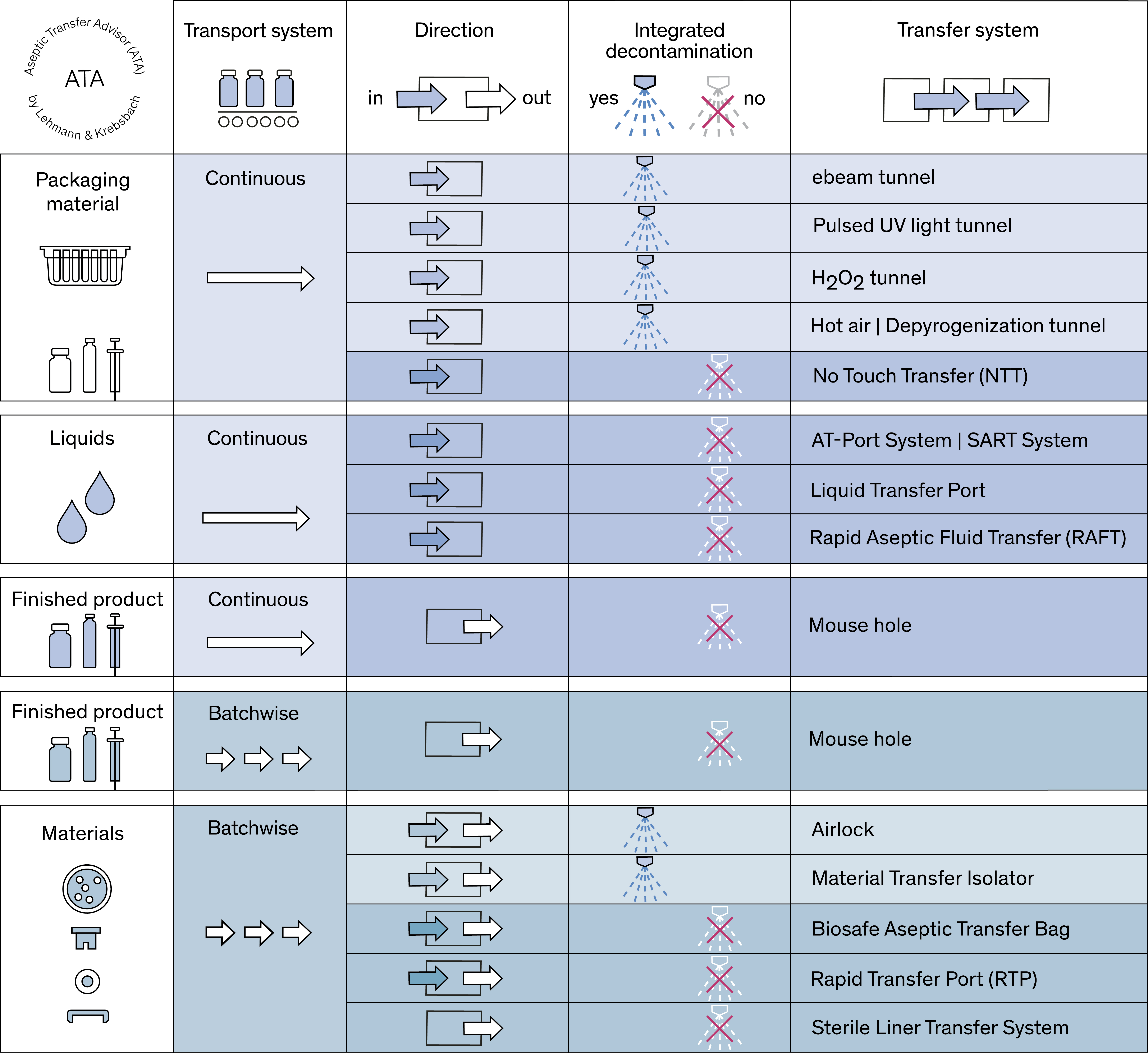
Fig. 1: Aseptic Transfer Advisor (ATA) (all figures provided by the authors)
The portfolio can be expanded as required with special solutions and custom-made products for very specific requirements.
Conclusion
The topic of material transfer is given great importance in Annex 1, particularly with regard to the required contamination control strategy. Today, there are a variety of different transfer options available, which are clearly presented in the Aseptic Transfer Advisor, ATA (see Fig. 1).
For all transfer processes that include a decontamination step, it should be noted that effective and efficient decontamination requires cleaned surfaces so that the decontamination agent can actually reach the microorganisms. This is also clearly emphasised in Annex 1 of the EC GMP Guideline.
The cleaning process prior to the biological decontamination step is of crucial importance; remaining residues can impair the effectiveness of the decontamination process [5,6]. Even though there are many outstanding technical achievements available that reduce the operator's influence to a minimum, it is still the case that ultimately it is the human being, with his or her aseptic behaviour, who influences whether the transfer process can proceed safely and reliably.
