Production facilities present many challenges with manual handling and material handling; moving a container to different locations and floor levels within the facility for each downstream process requirement safely. In GMP areas, these filled containers —be it a drum, bag, or IBC— could be anywhere from 1 kg upwards to 1,500 kg.
These challenges are potentially compounded by areas congested with other engineering apparatus (i.e. pipework or other process equipment) adding difficulty to the task of getting them to their final location.
To navigate the container to the next GMP downstream process step requires a precise operation and the capacity to reduce contamination risks. These are often mitigated by employing a grade of cleanroom around the process with strict control of personnel routes of ingress/egress and by defined gowning alongside other methods for the control of material ingress/egress.
Cross-contamination risk is also reduced by charging the contents of the container directly to the required process area with as minimal travel of the client’s excipient/intermediate/API product as possible, reducing the ‘product contact surfaces’ and thus minimising the risk of cross-contamination.
GMP production environments may also require strict specific materials that comply with client cleaning regimes that may be sporicidal sprays, acid or alkaline-based detergents, vapourised hydrogen peroxide, etc. This applies to everything in contact with the client’s product or going within a controlled environment.
Cross-contamination risk is also reduced by charging the contents of the container directly to the required process area
Other considerations for equipment manufacturers are that materials utilised should not shed particles or be thoroughly minimised as they can impact the particle count criteria and grading of the cleanroom.
PalPharma, a division of British materials handling company Palamatic, specialises in solutions to the material and manual handling challenges described above for GMP areas. The engineering approach ensures that designs are transferrable to other industries such as cosmetic, biotech, traditional pharma (oral solid dose, parenteral drug plants), fine chemical, and semiconductor, to name but a few.
The following case study describes an equipment engineering design. The solution is transferrable to other GMP-regulated industries requiring a standard of cleanliness.
Swiss client
The project emerged from a client who boasts 198,000 litres of biomedia preparation vessel capacity globally; one of the largest biotech companies worldwide. The company's portfolio of medicines to treat multiple sclerosis (MS) is at the forefront of research into new medicines for neurological conditions and rare genetic disorders. More than 7,000 employees are engaged in the business worldwide.
Located in Switzerland, the company's new facility was designed to produce up to ten metric tons of antibody per year to meet the rising global demand.
The project asked Palamatic for numerous post hoists to be designed as well as other ancillary items, supplied and commissioned within ISO Class 8 cleanrooms to allow client media preparation vessels at production platform level to be charged with powder product in varying containers.
The powders would initially arrive from a warehouse and be transported to the cleanroom area at ground level. It would then move up to the production level to that of the vessel with the agglomerated product to be vibrated in-situ on the hoist or to massage the drum to prevent solidification issues.

A multipurpose hoist coupled with a mobile vessel. Render by Palamatic
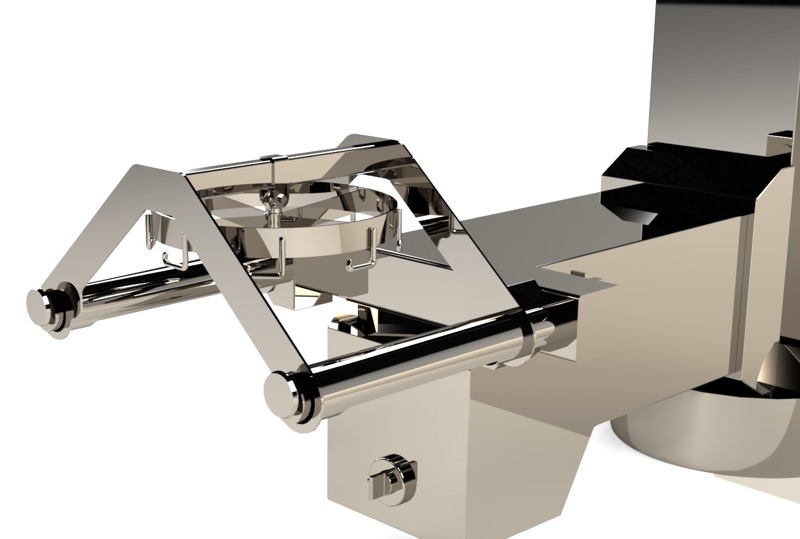
GMP-grade post hoists were chosen by the clients' engineering consultant, with novel changes suggested by Palamatic, to save the client overall process time while also increasing the hoists flexibility for other processes such as maintenance via a rapid "end effector" changeover. The end effector is a tool fixed onto the hoist that is used to grip, secure or retain the load to be moved.
Such hoists were also chosen for their ability to directly transfer to each vessel thus minimising loss, reducing the risk of cross-contamination in comparison to other engineering techniques such as vacuum transfer.
The post hoist method reduces the burden on a cleanroom via one complete charge, or by reducing the number of charges to a minimum
One might ask: why not just use more manageable 'pack sizes’ to charge the material to the vessel via operator ergonomics? It is important to note that the client is not always in control of the ‘pack sizes’ its team receives into the warehouse for varying reasons.
In this scenario, post hoists remove what would be the time-consuming need to sub-divide the product in another area typically done so the product is manageable by an operator to charge a vessel directly.
Sub-dividing would also run the risk of increased product loss if done internally; it would increase waste and expose the operator to dust nuisance/toxicity as well.
The post hoist method reduces the burden on a cleanroom via one complete charge, or by reducing the number of charges to a minimum and the need for a separate dispensary in relation to a sub-divide method.
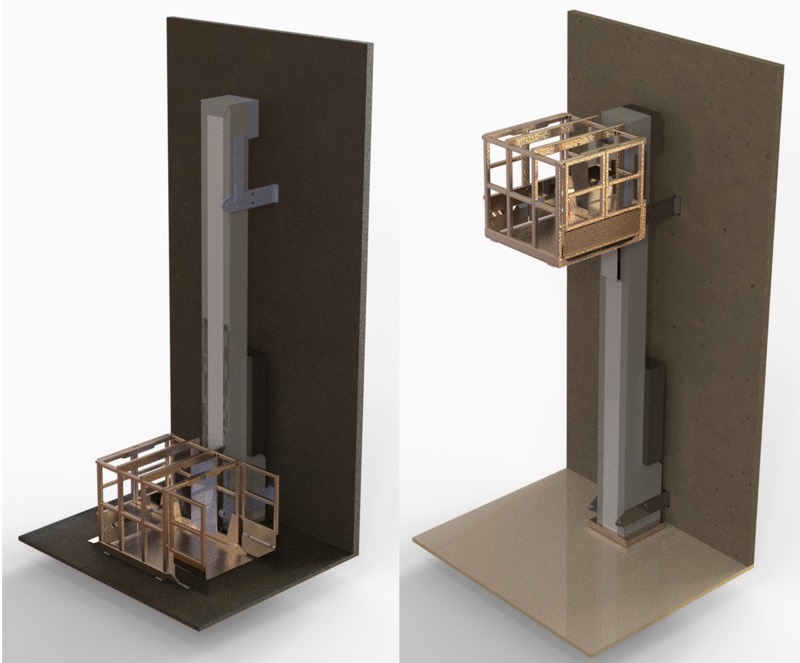
Lift positions. Render by Palamatic
Palamatic delivered a non-shedding, cleanable, GMP design as well as engineering advantages in this project in the shape of a novel hoist end effector that allowed various attachments (forks that can be adapted so the hoist can cater from IBCs to sacks, to maintenance platforms) to be quickly assembled to the ‘end effector’.
It is also important to note that due to the design of the outlet of each container that the hoist carries allows a controlled discharge of the product from the container, thus minimising impact to the cleanroom and loss of product from the batch.
Process applications
The project put to work 20 hoists across the following process applications:
- 14 PalPharmahoist platform, IBC & EZ-Biopac Supersack hoists with a 1000 kg SWL and 7m overall height, electrically powered.
A multipurpose hoist showing a ‘chandelier’ system for lifting DoverPac Ez-BioPac bags. Render by Palamatic
These hoists have a triple-purpose end effector that removes the need for end effector change over saving the client valuable time. Among other components, these units feature lifting ILC Dover EZ-BioPac super sacks; pins for lifting varying sized IBCs; and a platform on the end effector to allow maintenance operatives to take components safely and easily to the ground floor.
These pharma hoists, via a ground floor local operator panel (LOP), lift the super sack or IBC to a maximum of 6 m from floor level to a vessel platform level ‘slewing’ height where the hoist base would rotate allowing the EZ-BioPac/IBC PalPharmaIBC to hover over the manway of a media preparation vessel.
The hoist can then be lowered to the dust-tight connection to that of the manway. Where IBCs are to be discharged a sealing cap was utilised to contain any airborne dust nuisance.
Some of the 14 hoists supplied served two media preparation vessels.
- Four PalPharmahoist with ‘squeeze cone’ pneumatically powered: these hoists had to conform to ATEX regulations and had a ‘fork’ type end effector bottom with the top being a cone section.

A Squeeze Cone Hoist in drum inverted and upright positions. Render by Palamatic
Normally, when the drum squeeze cone is required the drums would be secured into the cradle and the fork-type bases automated lift would raise the drum into the squeeze cone assembly, securing it against a seal ready for inverted charging of the vessel.
The drum can then be lifted, rotated 180 degrees, slewed over a manway and then docked to it. The PalPharmahoist then fills the IBC with the required powder to be processed in the bioprocessing tanks.
- Two platform hoists: these hoists lift pallets to the production level.
- A ‘PalPharmassage drum massaging system to loosen the agglomerated product, electrically powered with a programmable logic controller (PLC) and human-machine interface (HMI). Recipes can be configured to suit varying diameter drums or type of drum; e.g. plastic or fibre.
Palamatic also supplied the following intermediate bulk containers:
- 20 off 100L IBC’s
- 6 off 300L IBC’s
- 4 off 500L IBC’s
Multipurpose trolleys have also been supplied to move an empty or full IBC around the factory, or to transport the EZ-BioPac end effector to another hoist; to adapt to the existing end effector fork assembly that resides on the hoist.
The mobile trolleys have a 304L stainless steel outer frame (non-product contact) assembly and 316L stainless steel product contact shell as well as FDA compliant gaskets. Sanitary caps and clamps are also provided.
Automatic discharge valves were employed on all IBCs with the utility to actuate each valve provided via the hoist’s ‘energy chain’ itself. A vibration feature was also provided on each drum and IBC hoist.
‘The EZ-BioPacs’ used in the project are from ILC Dover. These units bring fundamental advantages to traditional powder charging bags. They are easier to dispense via a much wider opening; they can be filled much quicker, notably, by around 71%.
Another benefit is a quicker discharge and recovery by reducing spill risk via a wider opening and a skirt, which captures any lost material that should have gone down the neck and also by having anti-static properties.
They can also connect directly to a tank that if overfilled is much easier to reduce the weight, compared to typical narrow neck bags, which are cumbersome and time-consuming.
Several orders in June 2016 paved the way to a seven-month delivery time frame. Palamatic had to meet a January date so that other cleanroom and construction works could be built around the ‘PalPharma’ hoists.
The site is nearly fully commissioned with site production starting shortly.
N.B. This article is exclusive to Cleanroom Technology magazine. The latest digital edition is available online.
